Oral nanoparticles for penetrating gastrointestinal mucus and epithelial cell barriers and preparation method and application of oral nanoparticles
A technology of epithelial cells and nanoparticles, applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the lack of effective drug delivery, weaken nanocarriers and Cell membrane affinity and other issues to achieve the effect of improving the ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A method for preparing oral nanoparticles for penetrating gastrointestinal mucus and epithelial cell barrier, characterized in that it comprises the following steps:
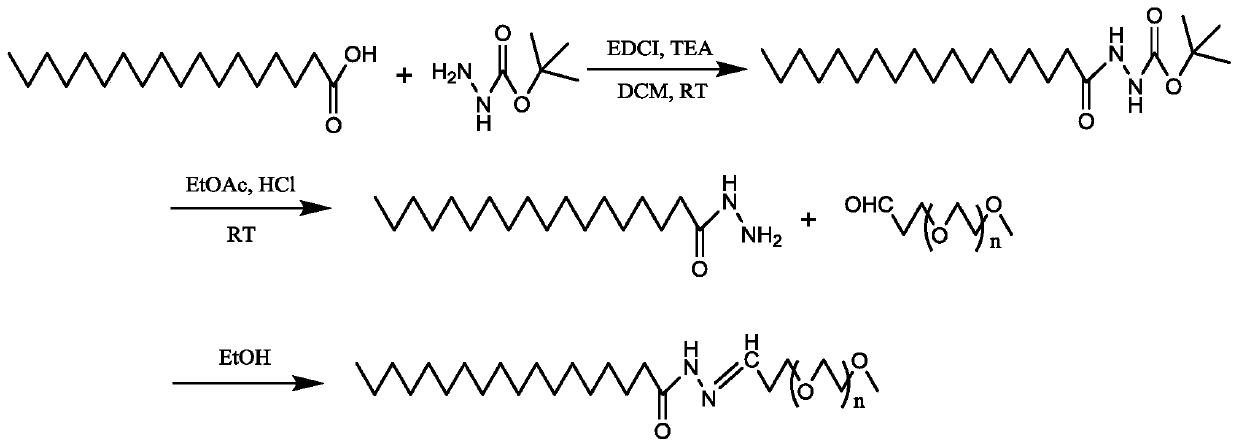
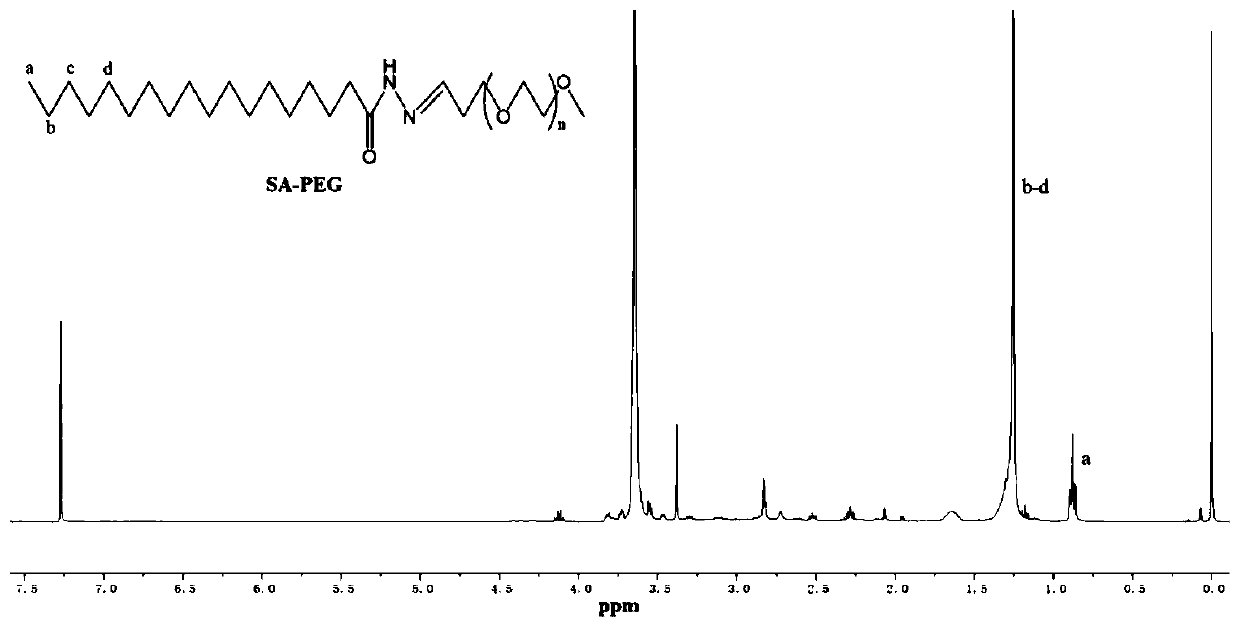
[0042] (1) Preparation of pH-responsive materials, the synthesis process is as follows figure 1 Shown:
[0043] A: Dissolve 2.50g of stearic acid (SA) in 100ml of dichloromethane (DCM), and add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride to the above solution (EDCI) 2.02g, triethylamine (TEA) 1ml and tert-butyl carbazate (boc-hydrazide) 1.39g, react at room temperature for 12h, after the reaction, wash with water 3 times, then wash 3 times with saturated brine, Then dried over anhydrous sodium sulfate, filtered, and the solvent was removed in vacuo to obtain 2.77 g of BOC-protected hydrazide SA;
[0044] B: Dissolve 1 g of BOC-protected hydrazide SA in 20 mL of ethyl acetate, add 2 drops of hydrochloric acid to the above solution, and react at room temperature for 2 h. After the reactio...
Embodiment 2
[0049] A method for preparing oral nanoparticles for penetrating gastrointestinal mucus and epithelial cell barrier, characterized in that it comprises the following steps:
[0050] (1) Preparation of pH-responsive materials:
[0051] A: Dissolve 1.10 g of stearic acid (SA) in 30 mL of dichloromethane (DCM), and add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride to the above solution (EDCI) 1.48g, triethylamine (TEA) 0.2mL and tert-butyl carbazate (boc-hydrazide) 0.61g, react at room temperature for 20h, after the reaction, wash with water 3 times, then wash 3 times with saturated brine , then dried over anhydrous sodium sulfate, filtered, and the solvent was removed in vacuo to obtain 1.31 g of BOC-protected hydrazide SA;
[0052] B: Dissolve 0.50 g of BOC-protected hydrazide SA in 15 mL of ethyl acetate, add 3 drops of hydrochloric acid dropwise to the above solution, and react at room temperature for 4 hours. After the reaction, the product is washed with deio...
Embodiment 3
[0057] A method for preparing oral nanoparticles for penetrating gastrointestinal mucus and epithelial cell barrier, characterized in that it comprises the following steps:
[0058] (1) Preparation of pH-responsive materials:
[0059] A: Dissolve 1.50 g of stearic acid (SA) in 20 mL of dichloromethane (DCM), and add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride to the above solution (EDCI) 1.72g, triethylamine (TEA) 0.2mL and tert-butyl carbazate (boc-hydrazide) 1.04g, react at room temperature for 24h, after the reaction, wash with water 3 times, then wash 3 times with saturated brine , then dried over anhydrous sodium sulfate, filtered, and the solvent was removed in vacuo to obtain 1.76 g of BOC-protected hydrazide SA;
[0060] B: Dissolve 0.50 g of BOC-protected hydrazide SA in 20 mL of ethyl acetate, add 3 drops of hydrochloric acid dropwise to the above solution, and react at room temperature for 6 hours. After the reaction, the product is washed with deio...
PUM
| Property | Measurement | Unit |
|---|---|---|
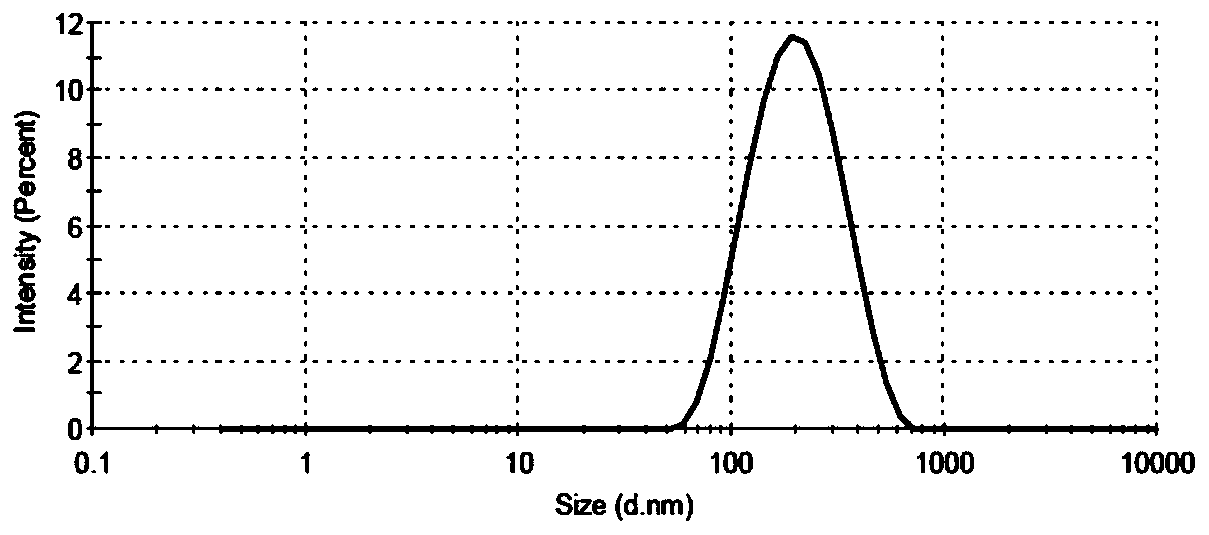
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com