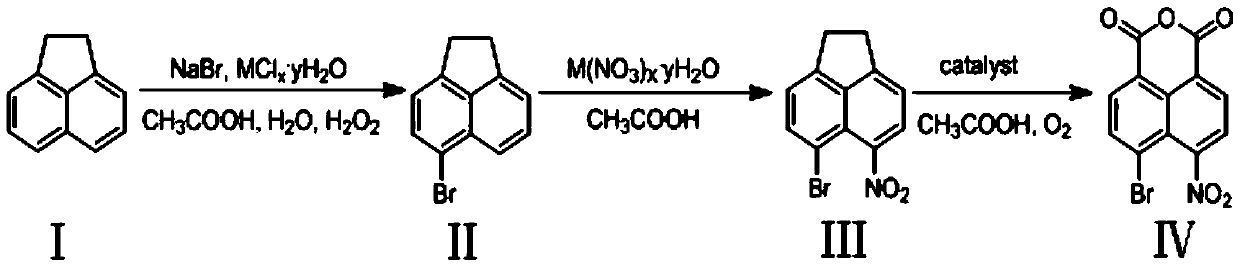
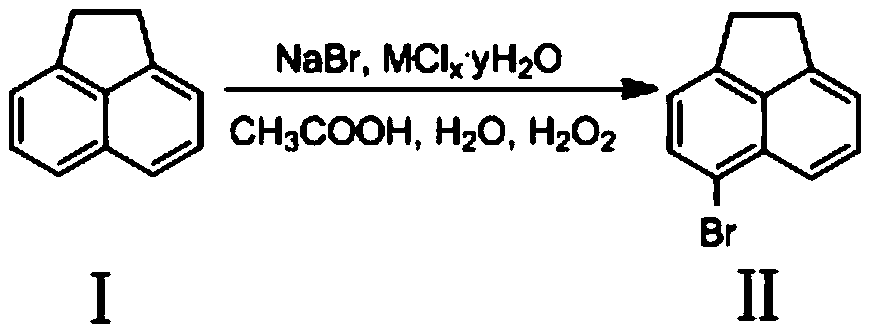Preparation method of 4-bromo-5-nitro-1,8-naphthalic anhydride
A technology of nitro and naphthalene anhydride, applied in the field of preparation of 4-bromo-5-nitro-1,8-naphthalene anhydride, which can solve the problems of difficulty in industrialization and application, narrow substrate universality, etc., and achieve cheap raw materials , Reduce production cost, good effect of atomic economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] Add 15.4g (0.0998mol) acenaphthene, 10.29g (0.1mol) sodium bromide, 2.3759 g (0.01mol) NiCl to the round bottom flask 2 ·6H 2 O (catalyst), 198mL glacial acetic acid and 99mL water, then dropwise added 204mL hydrogen peroxide and reacted at 20°C for 3 hours, then cooled the reaction solution to room temperature, suction filtered, and washed with 5-10mL H 2 The filter cake was washed with O and absolute ethanol, and the yellow product 5-bromoacenaphthene was obtained after vacuum drying with a yield of 92.4%. HRMS(ES+)C 12 h 10 Br([M+H]) + The theoretical value is 232.9966, and the measured value is 232.9964. As determined by HPLC, the purity of the target compound was 98.5%.
Embodiment 2
[0025] Except that catalyst is 1.7048g (0.01mol) CuCl in embodiment 2 2 2H 2 O, except that the temperature was controlled at 15° C. for 4 hours, other conditions and preparation steps were the same as in Example 1 to obtain a yellow product, 5-bromoacenaphthene, with a yield of 94.4%. HRMS(ES+)C 12 h 10 Br([M+H]) + The theoretical value is 232.9966, and the measured value is 232.9960. As determined by HPLC, the purity of the target compound was 98.3%.
Embodiment 3
[0027] Except that catalyst is 3.7258g (0.01mol) CeCl in embodiment 3 3 ·7H 2 O, except that the temperature was controlled at 20° C. for 3 hours, other conditions and preparation steps were the same as in Example 1 to obtain a yellow product, 5-bromoacenaphthene, with a yield of 97.5%. HRMS(ES+)C 12 h 10 Br([M+H])+ theoretical value 232.9966, measured value 232.9963. As determined by HPLC, the purity of the target compound was 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com