N-aryl-beta-carboline derivatives and application thereof
A technology of carbolin and its derivatives, which is applied in the field of N-aryl-β-carbolin derivatives, can solve the problem of insufficient anti-cardiomyocyte hypoxia-reoxygenation injury activity, which restricts the application of carbolin derivatives, Restrict the development of carbolin derivatives and other issues, achieve the effect of inhibiting the leakage of LDH in cells, improving the application, and reducing the toxicity of cardiomyocytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
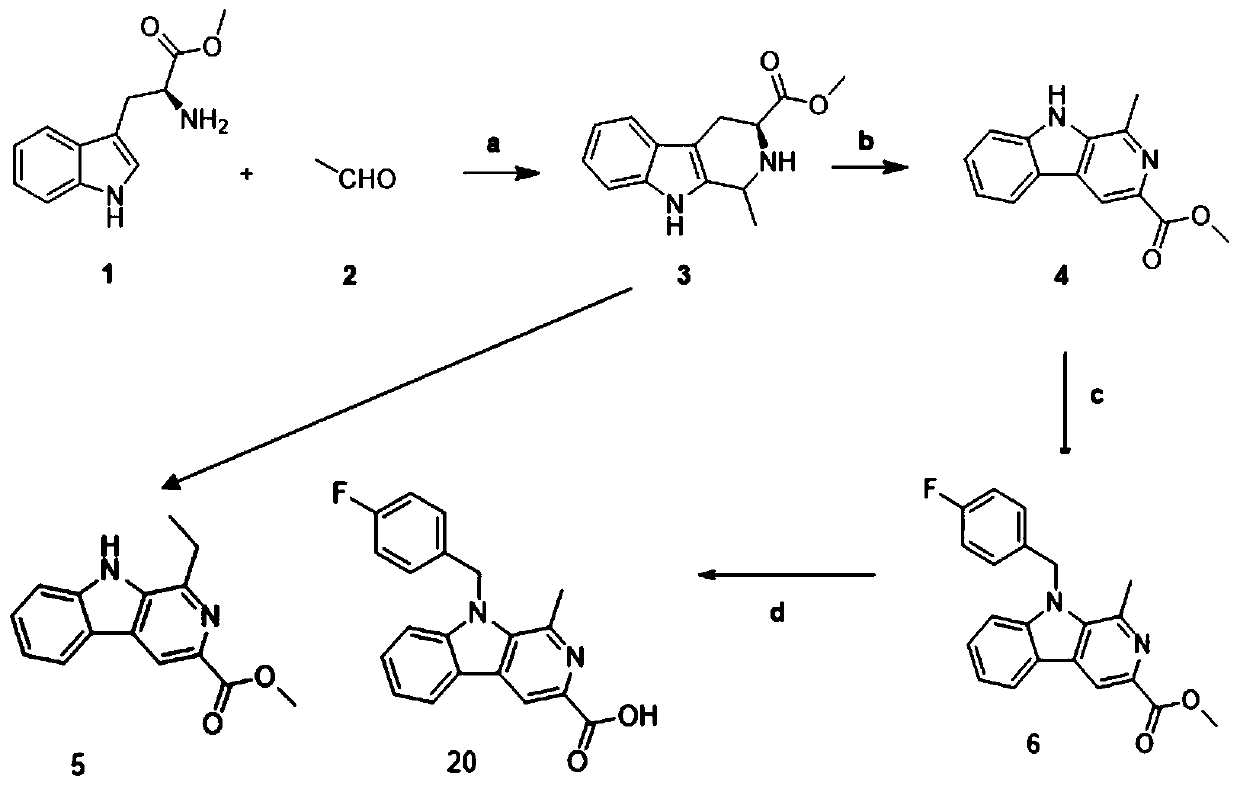
Embodiment 1
[0070] The preparation of embodiment 1 compound 6
[0071] Weigh dry, 4.58mmol of L-tryptophan methyl ester hydrochloride 1.0g, and 9.16mmol, 40% acetaldehyde 403.69mg, dissolve in 20mL of dichloromethane, then add 11.45mmol of trifluoroacetic acid 1.57g, room temperature The reaction was stirred under low temperature, and the reaction was detected by TLC until the reaction was completely terminated, 60 mL of water was added, extracted with dichloromethane, and the organic layer was spin-dried to obtain a crude product, which was purified by normal phase silica gel column chromatography to obtain compound 3 with a yield of 85%;
[0072] Weigh 1.0 g of dry 4.09 mmol of compound 3 into a 100 mL eggplant-shaped flask, add 15 mL of DMF solvent, and slowly add 8.19 mmol of KMnO at 0°C 4 1.29g, after the addition, continue to stir and react at 0°C for 1h, place at room temperature to continue the reaction, use TLC to detect the reaction until complete, stop the reaction, filter wit...
Embodiment 2
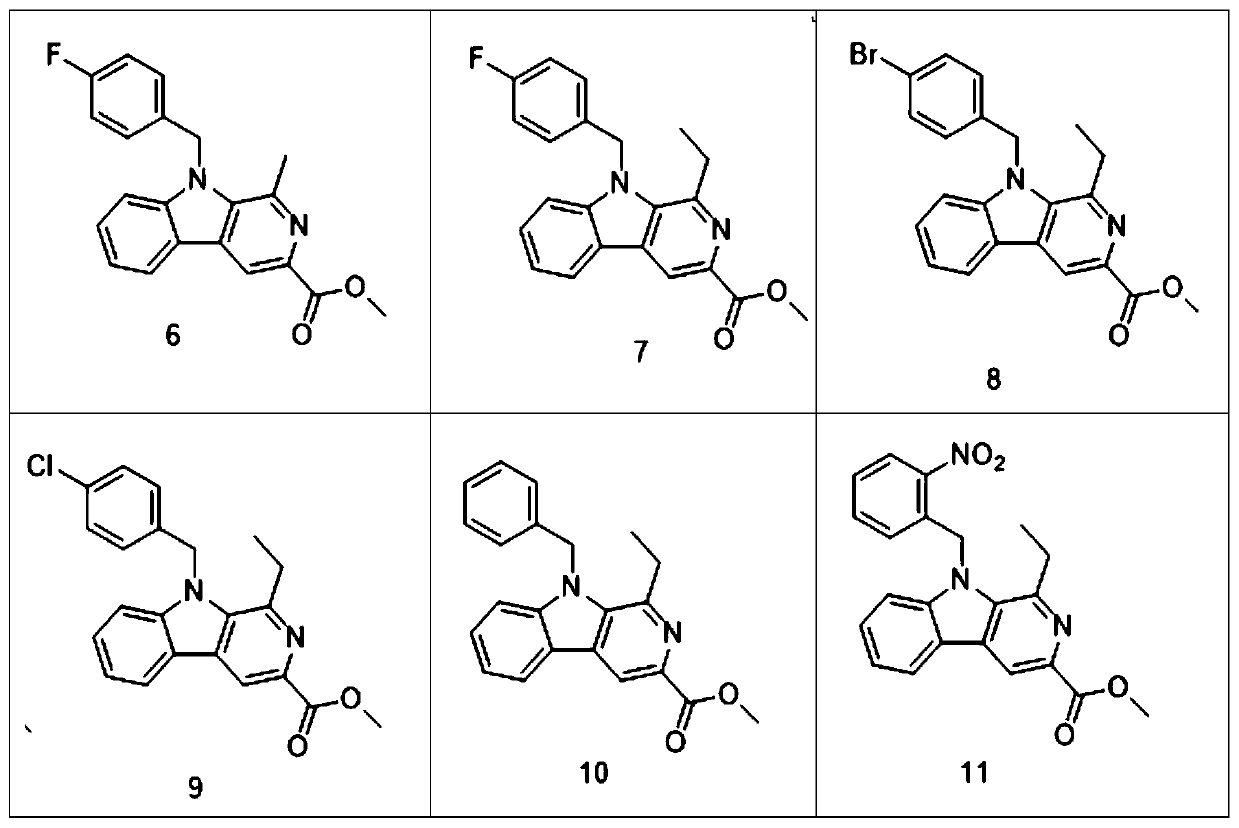
[0075] Compound 7——Preparation of N-(4-fluorobenzyl)-1-ethyl-3-methoxyl-β-carbolin
[0076] Referring to the preparation of compound 6, 1-methyl-3-methoxyacyl-β-carbolin was replaced with 1-ethyl-3-methoxyl-β-carbolin, and the yield was 63%;
[0077] Therefore, the preparation of 1-ethyl-3-methoxyl-β-carbolin (that is, compound 5) is to replace acetaldehyde in the preparation process of compound 4 with propionaldehyde.
Embodiment 3
[0079] Compound 8——Preparation of N-(4-bromobenzyl)-1-ethyl-3-methoxyacyl-β-carbolin
[0080] Referring to the preparation of compound 6, 4-fluorobromobenzyl was replaced by 4-bromobromobenzyl, and the yield was 59%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com