Injectable drug delivery depot of fulvestrant or derivative thereof, preparation method and application thereof
A technology of fulvestrant and its derivatives, which is applied in the field of medicine, can solve the problems of patient pain, low drug concentration, and unsatisfactory drug loading, and achieve the effect of reducing injection pain and reducing injection pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
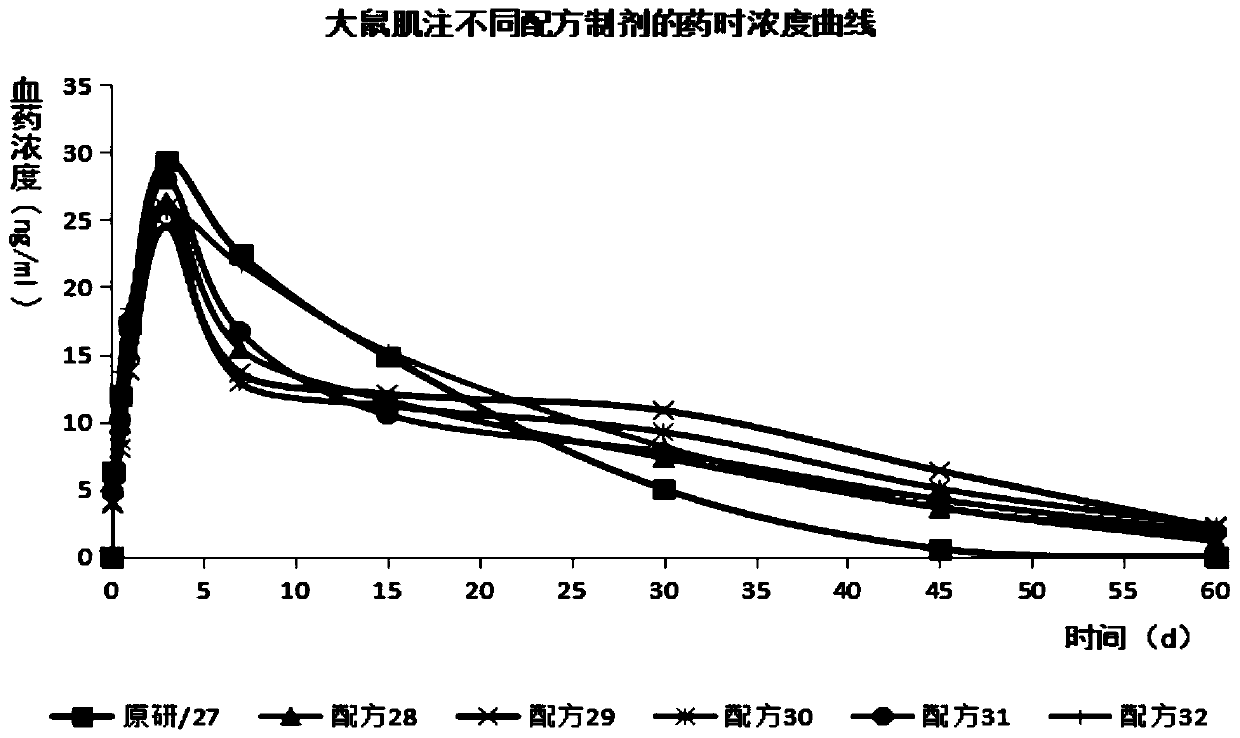
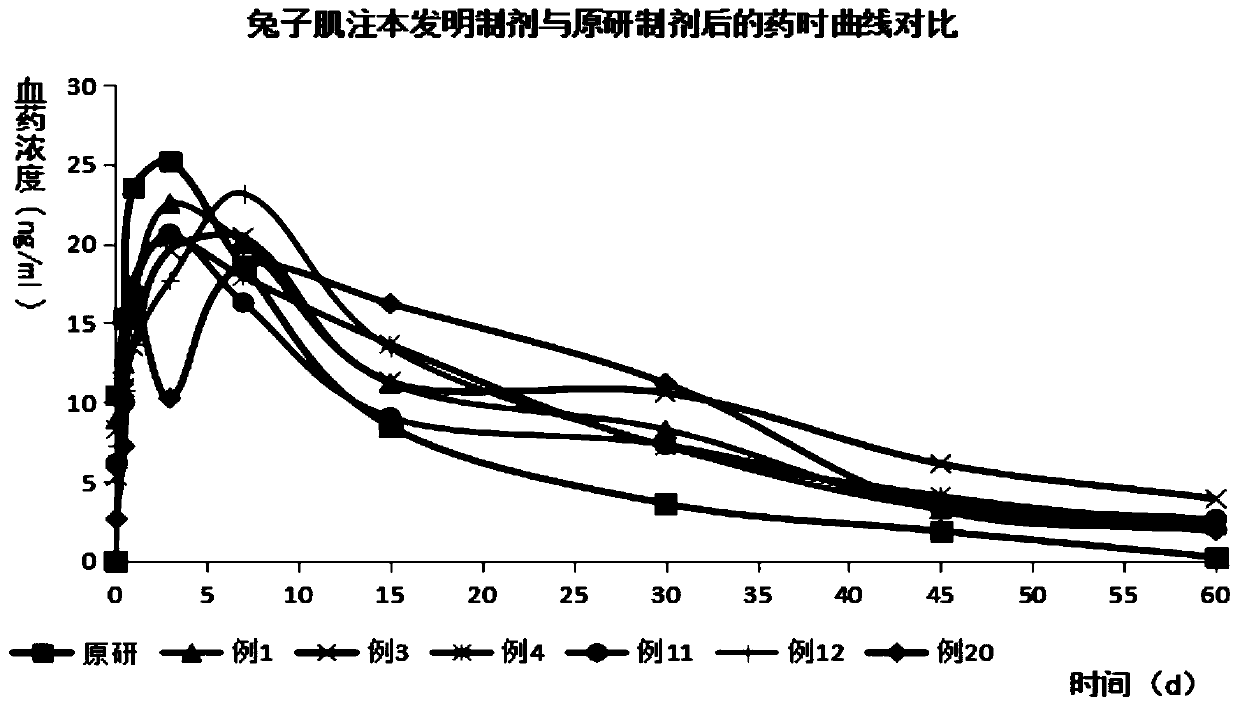
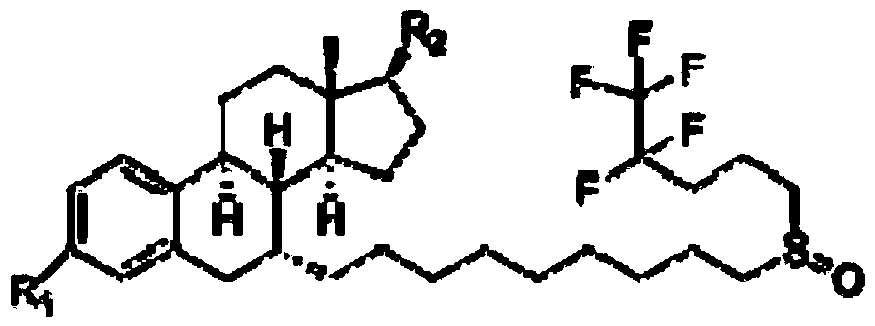
Image
Examples
Embodiment 1
[0112] This example is used to illustrate the preparation of the fulvestrant drug delivery depot, and the prescription is:
[0113]
[0114] Preparation scheme (method 3): 1) Weigh 100 mg of fulvestrant into a vial and sterilize it; 2) Weigh 100 mg of PLGA (80:20) with a molecular weight of 25000-35000, add 0.3 mL of NMP and 0.1 3) Mix 1 and 2 before use to obtain a drug delivery depot with a drug loading of about 180 mg / mL.
Embodiment 2
[0116] This example is used to illustrate the preparation of the fulvestrant drug delivery depot, and the prescription is:
[0117]
[0118] Preparation scheme (method 3): 1) Weigh 417.9 mg of fulvestrant-3-palmitate (equivalent to 300 mg of fulvestrant) and 1 mg of chlorobutanol according to the prescription, put them in a vial, and sterilize; 2) Take 0.4mL NMP, add 100mg PLGA (90:10) sequentially, molecular weight 10000-20000, ultrasonically dissolve, fill with nitrogen, and sterilize; 3) Mix 1 and 2 before use, and the drug loading amount is about 300mg / mL drug delivery depot.
Embodiment 3
[0120] This example is used to illustrate the preparation of the fulvestrant drug delivery depot, and the prescription is:
[0121]
[0122] Preparation (Method 3): 1) Weigh 500mg of fulvestrant and sterilize; 2) Take 0.8mL of NMP, add 350mg of PLGA (50:50) to dissolve, then add 0.1mL of benzyl alcohol and 50mg of vitamin E acetate in sequence , mixed, filled with nitrogen, and sterilized; 3) before use, the two were mixed to obtain a drug delivery depot with a drug loading of about 250 mg / mL.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com