Collagen dressing capable of realizing sustained and control release of antibiotics and preparation method of collagen dressing
A technology of antibiotics and collagen, applied in medical science, absorbent pads, bandages, etc., can solve the problems of materials that cannot be applied to infection, fast degradation speed, low mechanical strength, etc., to improve liquid absorption, adhesion, and anti-degradation performance Low, low mechanical strength effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
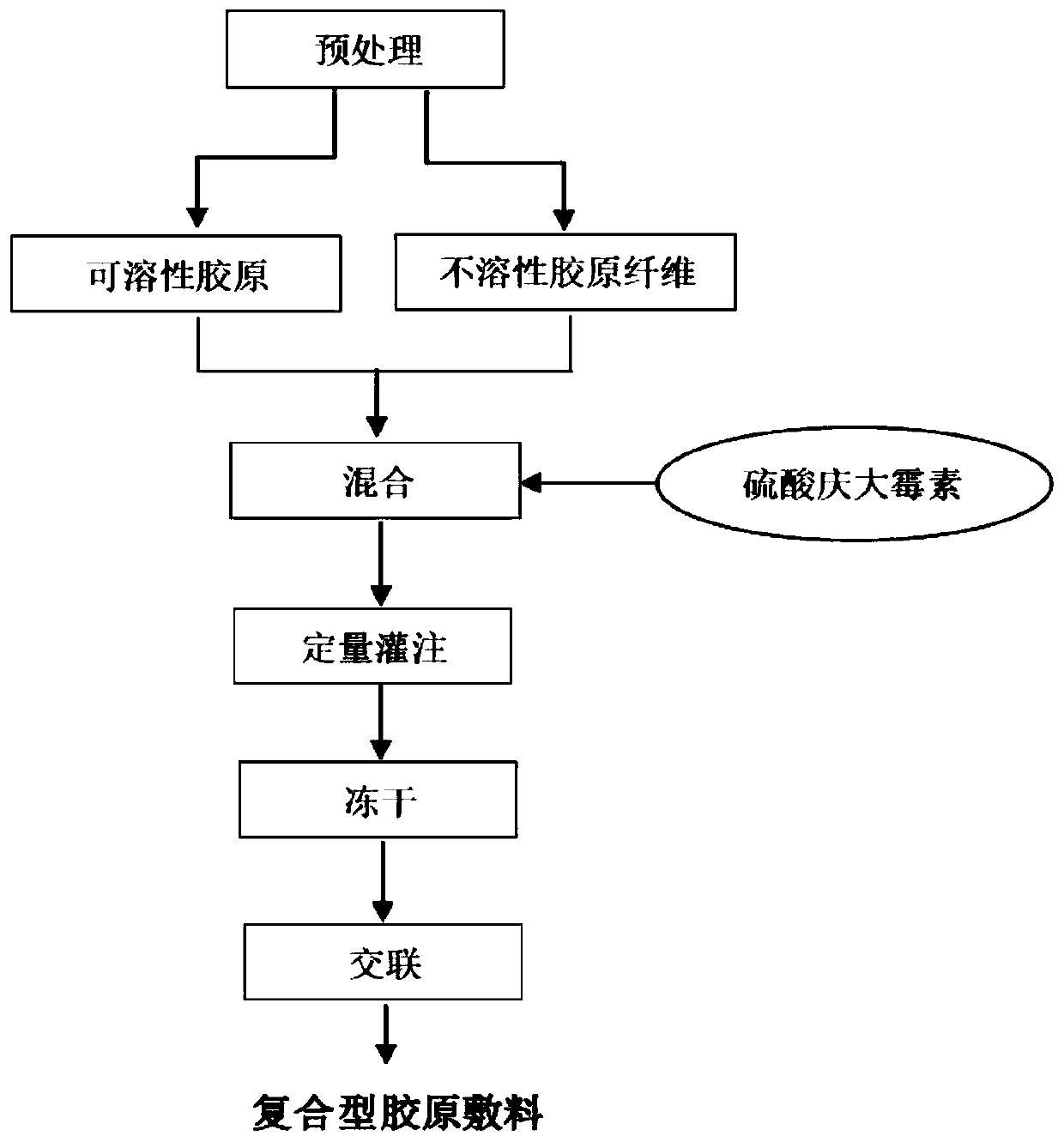
[0055] A preparation method of a collagen dressing capable of slow and controlled release of antibiotics, the steps of the method are as follows:
[0056] Preparation of insoluble collagen fibers: slice the tendon, wash, pulverize, degrease and sterilize. Weigh 100 g of pretreated beef tendon slices, put them into 500 mL of 1% NaOH aqueous solution, mix them evenly, and soak them at 8°C for 5 days. After soaking, wash with 0.1% acetic acid aqueous solution until white flocs are precipitated. Put the white floc into 500mL of 4% hydrochloric acid solution, and slowly stir for 20h at 10°C. After acid treatment, wash with purified water until the weight of the floc is 2.5 times that of the beef tendon sheet, and finally the floc is crushed into uniform white silky short fibers with a pulverizer, and the dry matter content is measured to obtain its The dry matter content was 12.86%.
[0057] The preparation of the collagen dressing that can release antibiotic slowly: Weigh 62.21...
Embodiment 2
[0059] A preparation method of a collagen dressing capable of slow and controlled release of antibiotics, the steps of the method are as follows:
[0060] Preparation of insoluble collagen fibers: slice the tendon, wash, pulverize, degrease and sterilize. Weigh 100 g of pretreated beef tendon slices, put them into 500 mL of 1.5% NaOH aqueous solution, mix them evenly, and soak them at 9° C. for 6 days. After soaking, wash with 0.12% acetic acid aqueous solution until white flocs are precipitated. Put the white floc into 500mL 5% hydrochloric acid solution, and stir slowly for 19h at 10°C. After the acid treatment, wash with purified water until the weight of the flocs is twice that of the beef tendon slices. Finally, the flocs are crushed into uniform white filamentous short fibers with a pulverizer, and the dry matter content is measured. The dry matter content was 10.85%.
[0061] The preparation of the collagen dressing that can release antibiotic slowly: Weigh 41.47g in...
Embodiment 3
[0063] A preparation method of a collagen dressing capable of slow and controlled release of antibiotics, the steps of the method are as follows:
[0064] Preparation of insoluble collagen fibers: slice the tendon, wash, pulverize, degrease and sterilize. Weigh 100 g of pretreated beef tendon slices, put them into 500 mL of 2% NaOH aqueous solution, mix well, and soak for 5 days at 10° C. After soaking, wash with 0.15% acetic acid aqueous solution until white flocs are precipitated. Put the white floc into 500mL of 4% hydrochloric acid solution, and slowly stir for 20h at 10°C. After the acid treatment, wash with purified water until the weight of the floc is 3 times that of the beef tendon slices, and finally the floc is crushed into uniform white silky short fibers with a pulverizer, and its dry matter content is measured. The dry matter content was 13.12%.
[0065] The preparation of the collagen dressing that can release antibiotic slowly: Weigh 102.90g insoluble collag...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Internal aperture | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com