Preparation method of rare earth fluoride
A technology of rare earth fluorides and rare earth elements, applied in the direction of rare earth metal fluorides, rare earth metal halides, etc., can solve the problems of complex steps, human hazards, and difficulty in hydrogen fluoride tail gas treatment, and achieve high purity and low water and oxygen content in products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
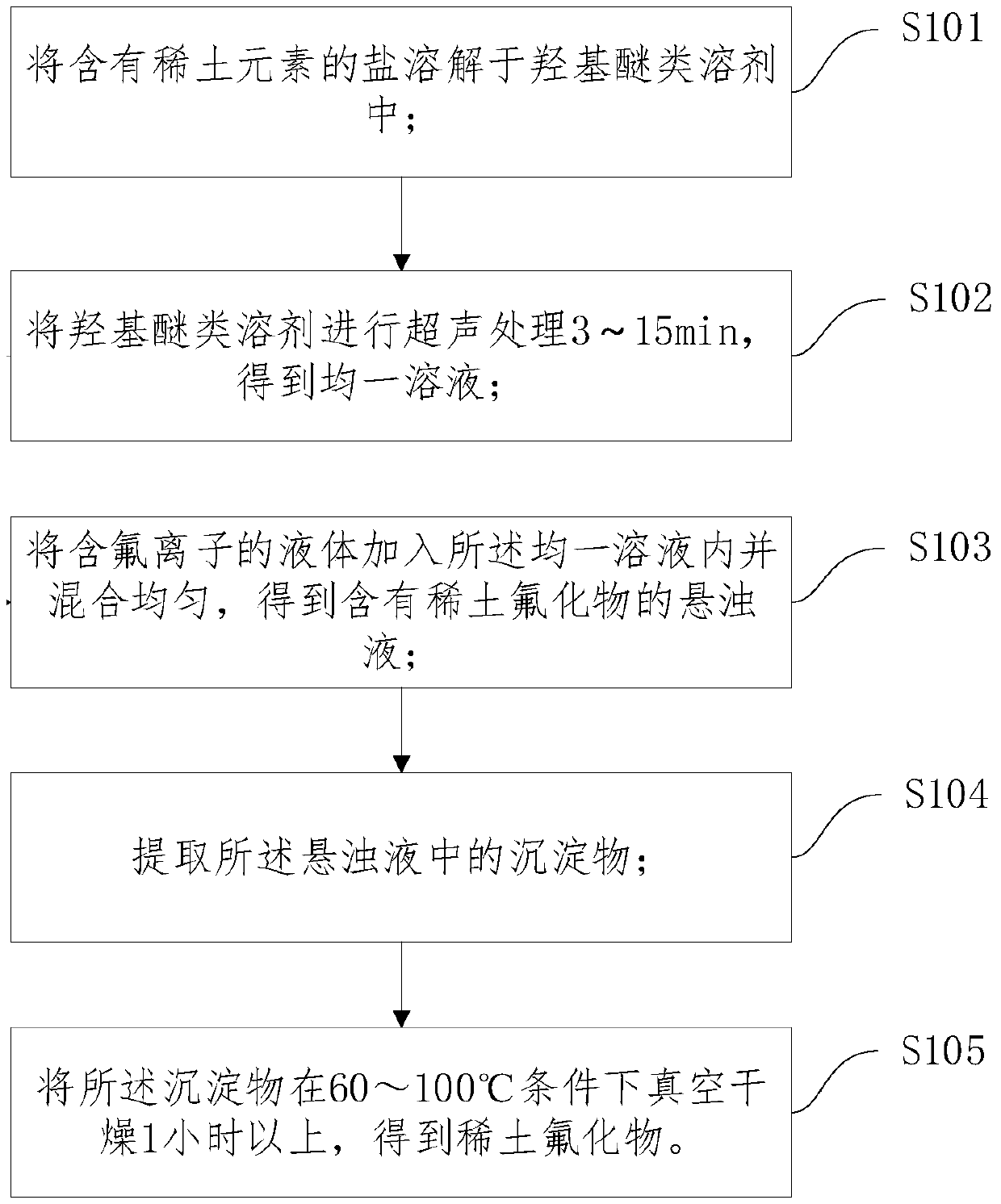
[0023] Such as figure 1 As shown, the steps of the method for preparing rare earth fluorides include: step S101 to step 105:
[0024] Step S101, dissolving the salt containing rare earth elements in a hydroxy ether solvent.
[0025] Among them, the salt containing rare earth elements is rare earth chloride salt, rare earth nitrate or rare earth sulfate.
[0026] Optionally, the salt containing rare earth elements is DyCl 3 , NdCl 3 、LaCl 3 or La(NO 3 ) 3 one or more of.
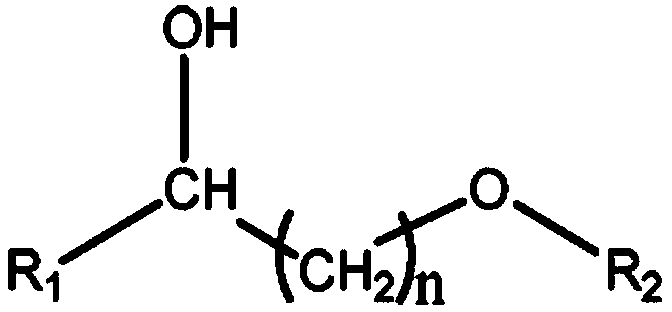
[0027] Specifically, the structural formula of the hydroxy ether solvent is:
[0028]
[0029] Wherein, R1 is any one of hydrogen, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl and tert-butyl; R2 is hydrogen, methyl, ethyl, n-propyl , isopropyl, n-butyl, isobutyl and tert-butyl; 1≤n≤10, and n is a positive integer.
[0030] In an optional embodiment, the hydroxy ether solvent is ethylene glycol or 2-hydroxypropyl methyl ether.
[0031] Step S102, ultrasonically treating the hydroxy ether ...
Embodiment 1
[0048] 36g of NdCl 3Dissolve in 500mL ethylene glycol, and sonicate it for 10min to obtain a homogeneous solution. Add 25 mL of 1-ethyl-3-methylimidazolium tetrafluoroborate dropwise into the homogeneous solution, stir with a glass rod during the dropwise addition, and stir for 10 minutes after the addition to obtain a suspension. The suspension was suction-filtered to obtain 30 g of a precipitate, which was washed with water and ethanol respectively, and then vacuum-dried at 60° C. for 24 hours to obtain 25 g of a reaction product.
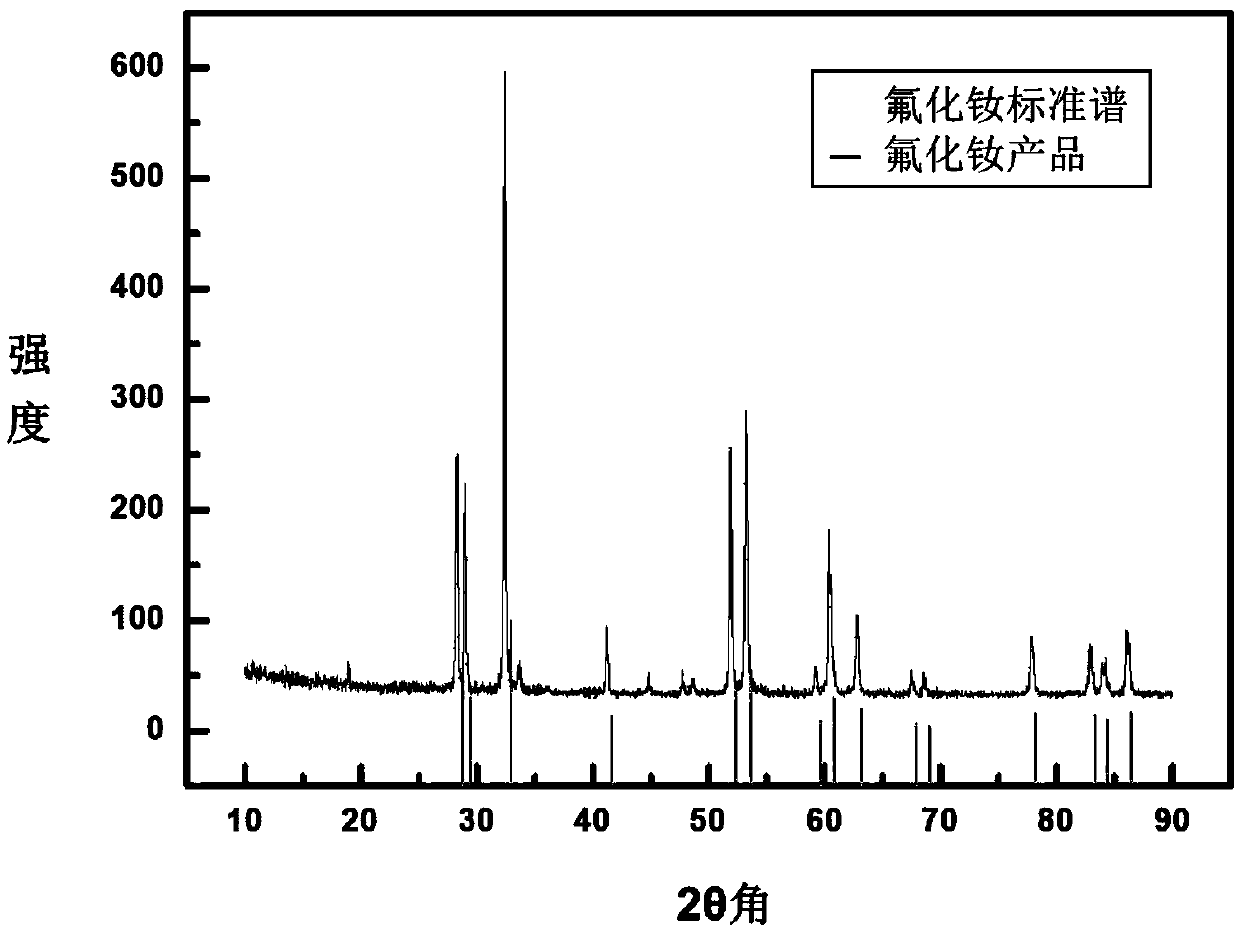
[0049] figure 2 is the XRD pattern of the reaction product.
[0050] Such as figure 2 Shown, where the black line is NdF 3 XRD pattern, the gray line is the XRD pattern of the reaction product. After comparison, the precipitate is NdF 3 . It can be seen that the method of the present application is used to prepare rare earth fluorides, and the yield is relatively high. The oxygen content of the reaction product was analyzed by an oxygen...
Embodiment 2
[0052] 49g of LaCl 3 Dissolve in 700 mL 2-hydroxypropyl methyl ether, and sonicate it for 5 min to obtain a homogeneous solution. Add 45mL of 1-butyl-3-methylimidazolium hexafluorophosphate dropwise into the homogeneous solution, and stir with magnetic force during the dropwise addition. After complete addition, stirring for 10 minutes resulted in a suspension. The suspension was suction-filtered to obtain 35 g of a precipitate, which was washed with water and ethanol respectively, and then vacuum-dried at 100° C. for 18 hours to obtain 28 g of a reaction product.
[0053] By performing X-ray diffraction on the reaction product, the XRD pattern of the reaction product is obtained, which proves that the reaction product is LaF 3 . The oxygen content of the reaction product was analyzed by an oxygen and nitrogen analyzer, and the oxygen content was 60 ppm. Illustrate adopting the method that the embodiment of the present invention provides to prepare LaF 3 , the oxygen cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com