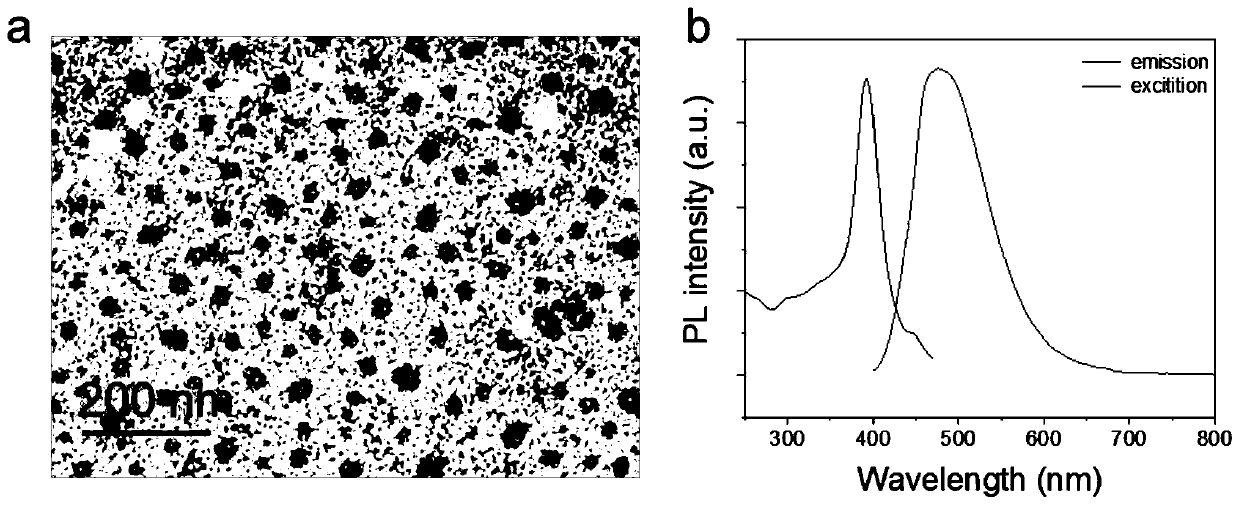
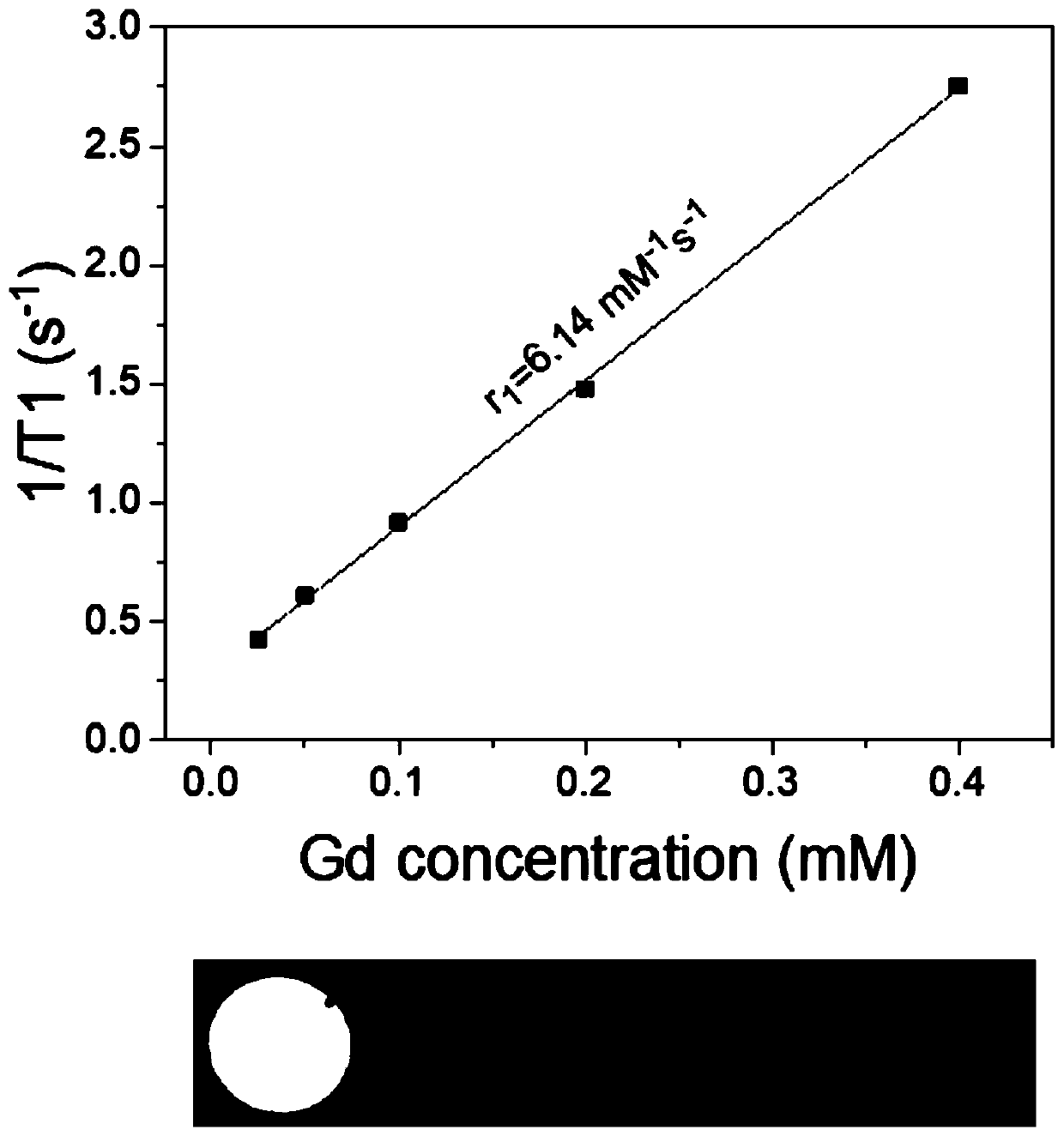
Polymer with targeted nuclear magnetic resonance imaging and fluorescence imaging functions, preparation method and application
A nuclear magnetic resonance and fluorescence imaging technology, which is used in nuclear magnetic resonance/magnetic resonance imaging contrast agents, general/multifunctional contrast agents, preparations for in vivo tests, etc. Clinical and market application potential, good application prospects, and the effect of excellent biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] In this embodiment, the targeted nuclear magnetic resonance imaging and fluorescence imaging polymers were prepared according to the following steps:
[0044] Step 1: Dissolve 1.3g of hydroxyethyl methacrylate and 2g of triethylamine in 20mL of dichloromethane, add 2.2g of bromoacetyl bromide, stir at 0°C for 12h, and separate the resulting product through a silica gel column (eluent is acetic acid ethyl ester and n-hexane), spin-dried, then mixed with 3.3g 1,4,7,10-tetraazacyclododecane-1,4,7-tri-tert-butyl triacetate and 2.5g potassium carbonate in acetonitrile reaction in China for 12 hours, the resulting product was filtered and extracted to obtain the product tri-tert-butyl methacrylate-1,4,7,10-tetraazacyclododecane-1,4,7-triacetate ;
[0045] Step 2: Dissolve 28 mg of 4-cyano-4-(thiobenzoyl)valeric acid in 10 mL of 1,4-dioxane, add it to a polymer bottle, and then add 1.2 g of polyethylene glycol methacrylate Esters, 0.55g hydroxyethyl methacrylate-1,4,7,10-tet...
Embodiment 2
[0056] In this embodiment, the targeted nuclear magnetic resonance imaging and fluorescence imaging polymers were prepared according to the following steps:
[0057] Step 1: Dissolve 3 g of hydroxyethyl methacrylate and 5 g of triethylamine in 50 mL of dichloromethane, add 5 g of bromoacetyl bromide, stir at 0° C. for 6 h, and separate the resulting product through a silica gel column (eluent is ethyl acetate and n-hexane), spin-dried, then reacted with 6g of 1,4,7,10-tetraazacyclododecane-1,4,7-tri-tert-butyl triacetate and 5g of potassium carbonate in acetonitrile for 6h, the obtained After the product is filtered and extracted, the product tri-tert-butyl methacrylate-1,4,7,10-tetraazacyclododecane-1,4,7-triacetate is obtained;
[0058] Step 2: Dissolve 56 mg of 4-cyano-4-(thiobenzoyl)valeric acid in 15 mL of 1,4-dioxane, add it to a polymer bottle, and then add 2 g of polyethylene glycol methacrylate , 1g of hydroxyethyl methacrylate-1,4,7,10-tetraazacyclododecane-1,4,7-tr...
Embodiment 3
[0066] In this embodiment, the targeted nuclear magnetic resonance imaging and fluorescence imaging polymers were prepared according to the following steps:
[0067] Step 1: Dissolve 10 g of hydroxyethyl methacrylate and 15 g of triethylamine in 150 mL of dichloromethane, add 17 g of bromoacetyl bromide, stir at 0° C. for 24 h, and separate the resulting product through a silica gel column (eluent is ethyl acetate and n-hexane), spin-dried, then reacted with 8g of 1,4,7,10-tetraazacyclododecane-1,4,7-tri-tert-butyl triacetate and 8g of potassium carbonate in acetonitrile for 24h. After the obtained product is filtered and extracted, the product tri-tert-butyl methacrylate-1,4,7,10-tetraazacyclododecane-1,4,7-triacetate is obtained;
[0068] Step 2: Dissolve 100mg of 4-cyano-4-(thiobenzoyl)valeric acid in 20mL of 1,4-dioxane, add it to a polymer bottle, and then add 9g of polyethylene glycol methacrylate , 4g of hydroxyethyl methacrylate-1,4,7,10-tetraazacyclododecane-1,4,7-tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com