Aluminum oxide coated hafnium/nitrogen co-doped lithium iron phosphate positive electrode material and preparation method thereof
A technology of lithium iron phosphate and positive electrode material, applied in chemical instruments and methods, phosphates, phosphorus oxyacids, etc., can solve the problems of low ion conductivity and electron conductivity of lithium iron phosphate, low rate discharge and other performance and production costs. Satisfy and hinder the large-scale application of materials, etc., to achieve the effect of improving electrochemical performance, huge economic value and environmental protection value, and low manufacturing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
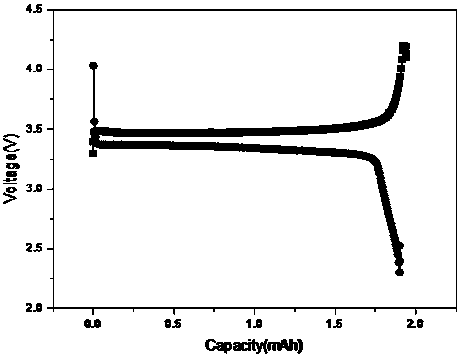
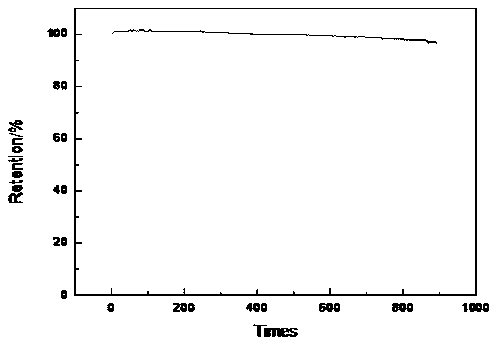
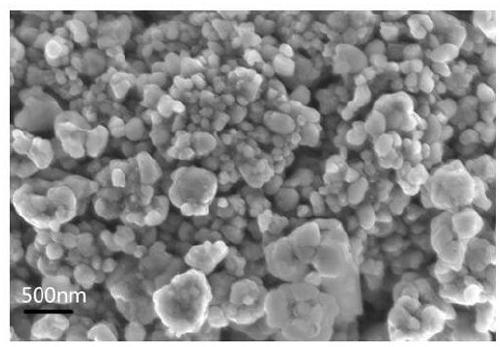
Image
Examples
Embodiment 1
[0028] At room temperature, weigh Hf(NO 3 ) 4 , lithium carbonate, diammonium hydrogen phosphate, and ferrous sulfate heptahydrate were added to deionized water to disperse and stir to prepare a dispersion liquid, wherein the stirring time was selected to be 10 hours, and the stirring rate was 1500 rpm. The obtained dispersion liquid was evaporated to dryness by rotary evaporation, the rotary evaporation temperature was 70° C., and the rotational speed was 100 rpm. Afterwards, the dried mixture was calcined in a tube furnace at a temperature of 500°C for 10 hours at a heating rate of 1°C / min to obtain a lithium iron phosphate precursor. The obtained lithium iron phosphate precursor is placed again in the mixed solution of aluminum nitrate, urea and glucose, the molar ratio of aluminum nitrate, urea and glucose is 0.1:5:1, the quality of lithium iron phosphate precursor and aluminum nitrate, The ratio of the mass sum of urea and glucose is 1:2, the rapid stirring speed is 120...
Embodiment 2
[0030] At room temperature, weigh HfCl according to the molar ratio of 0.2:1:1:1 4, lithium nitrate, diammonium hydrogen phosphate, and ferrous sulfate heptahydrate were added to deionized water to disperse and stir to prepare a dispersion liquid, wherein the stirring time was selected to be 20 hours, and the stirring rate was 900 rpm. The obtained dispersion liquid was evaporated to dryness by rotary evaporation, the rotary evaporation temperature was 70° C., and the rotational speed was 100 rpm. Afterwards, the dried mixture was calcined in a tube furnace at a temperature of 600°C for 8 hours at a heating rate of 2°C / min to obtain a lithium iron phosphate precursor. The obtained lithium iron phosphate precursor is placed back in the mixed solution of aluminum nitrate, urea and glucose, the molar ratio of aluminum nitrate, urea and glucose is 0.15:5:1, the quality of lithium iron phosphate precursor and aluminum nitrate, The ratio of the mass sum of urea and glucose is 1:2, ...
Embodiment 3
[0032] At room temperature, weigh HfCl according to the molar ratio of 0.15:1:1:1 4 , lithium nitrate, phosphoric acid, and ferric chloride were added into deionized water to disperse and stir to prepare a dispersion liquid, wherein the stirring time was selected to be 20 hours, and the stirring rate was 900 rpm. The obtained dispersion liquid was evaporated to dryness by rotary evaporation, the rotary evaporation temperature was 70° C., and the rotational speed was 100 rpm. Afterwards, the dried mixture was calcined in a tube furnace at a temperature of 700°C for 5 hours at a heating rate of 3°C / min to obtain a lithium iron phosphate precursor. The obtained lithium iron phosphate precursor is placed back in the mixed solution of aluminum nitrate, urea and glucose, the molar ratio of aluminum nitrate, urea and glucose is 0.15:6:1.5, the quality of lithium iron phosphate precursor is the same as that of aluminum nitrate, The ratio of the mass sum of urea and glucose is 1:2, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com