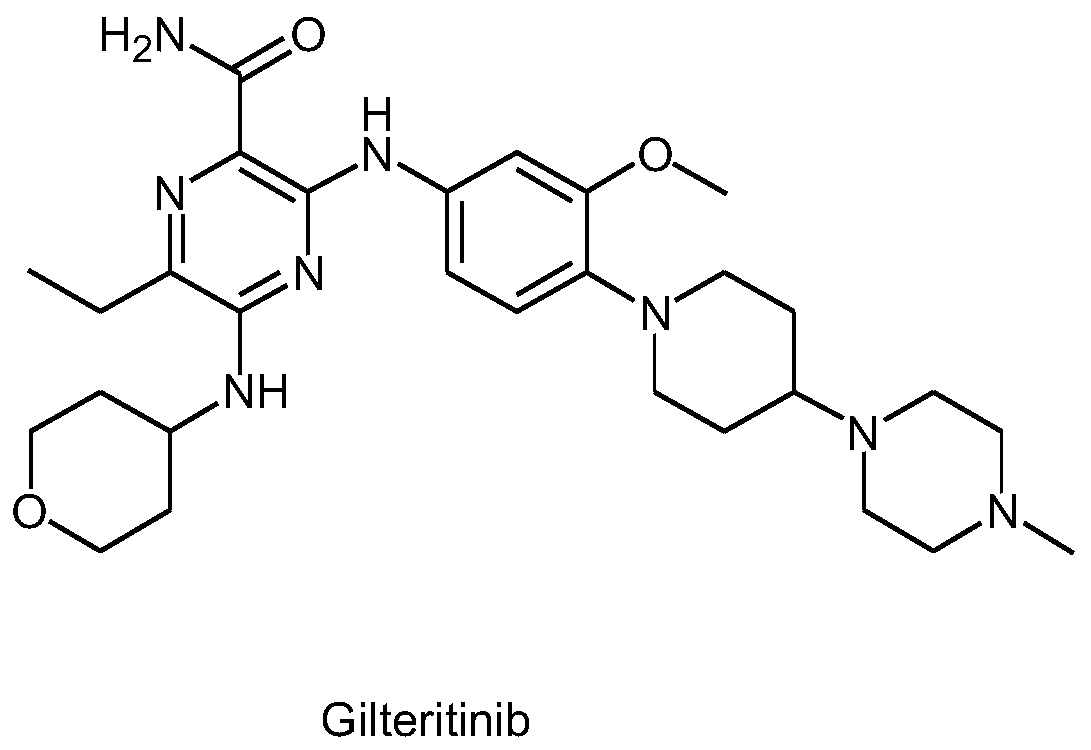
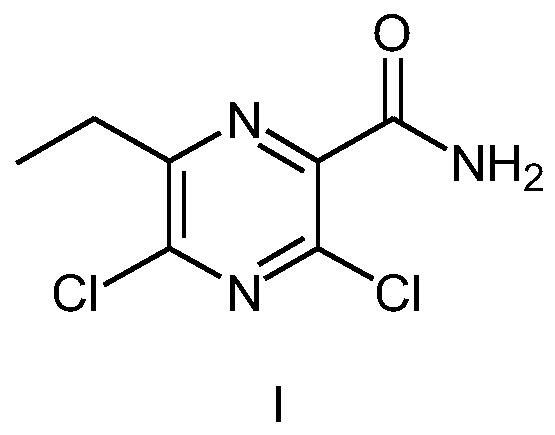
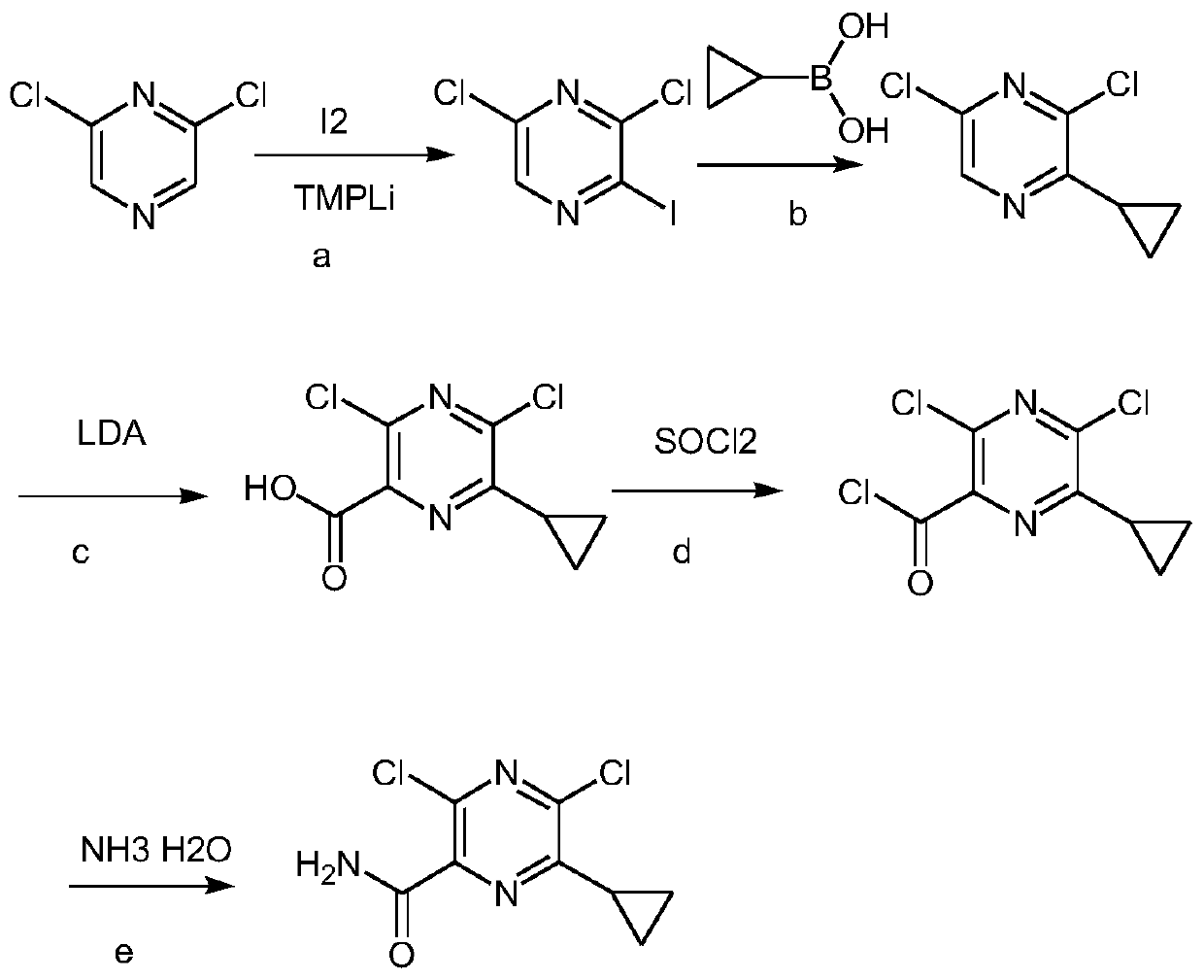
Preparation method of Gilteritinib key intermediate
A compound and solvent technology, applied in the field of drug synthesis, can solve the problems of heavy metal pollution, inability to provide large-scale production, and high production costs, and achieve the effects of avoiding metal lithium reagents, good methodological significance, and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A method for preparing 3,5-dichloro-6-ethylpyrazinecarboxamide (compound I), such as Figure 4 shown, including the following steps:
[0028] (1) Preparation of compound III from compound II: add 600 ml of 1N sodium hydroxide solution to a 1000 ml reaction flask, add 100 g of ethyl propionoacetate dropwise into the reaction flask in an ice-water bath, after the addition is complete, warm to room temperature and stir for 12 h As above, dissolve 52 g of sodium nitrite in 200 ml of water, drop it into the reaction system, and add 200 ml of 1N hydrochloric acid dropwise at 5 °C, complete the dropwise addition within 1 hour, stir for 2-3 hours, and dilute the reaction solution with acetic acid Ethyl ester (300 mL*2) was extracted, the organic phase was washed with 500 ml of saturated brine, dried over anhydrous sodium sulfate, and spin-dried to obtain 65 g of off-white solid with a yield of 91% and a purity of 97%.
[0029] ESI-MS, m / z (%): 102 (M + H )+.
[0030] (2) Prep...
Embodiment 2
[0039] A method for preparing 3,5-dichloro-6-ethylpyrazinecarboxamide (compound I), such as Figure 4 shown, including the following steps:
[0040] (1) Preparation of compound III from compound II: add 600ml of 1N potassium hydroxide to a 1000ml reaction bottle, cool down to 0°C in an ice-water bath, add 100g of ethyl propionyl acetate dropwise, return to room temperature and stir for 12h, dissolve 52g of sodium nitrite in water Add 200ml of acetic acid dropwise to the reaction system, keep at 5°C, add 200ml of acetic acid dropwise, complete the dropwise addition within 1h, stir for 2-3h, add 300ml of ethyl acetate to the reaction solution for extraction twice, use saturated brine for the ethyl acetate phase 500ml was washed, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain 65g of off-white solid with a yield of 91%.
[0041] ESI-MS, m / z(%):102 (M + H ) + .
[0042] (2) Preparation of compound V from compound III: add 100...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com