Combined drug for treating diabetes mellitus and complications and pharmaceutical composition of combined drug
A composition and diabetes technology, applied in drug combinations, urinary system diseases, active ingredients of heterocyclic compounds, etc., can solve problems such as effective population and long-term curative effect limitations, complication prevention and control effect differences, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
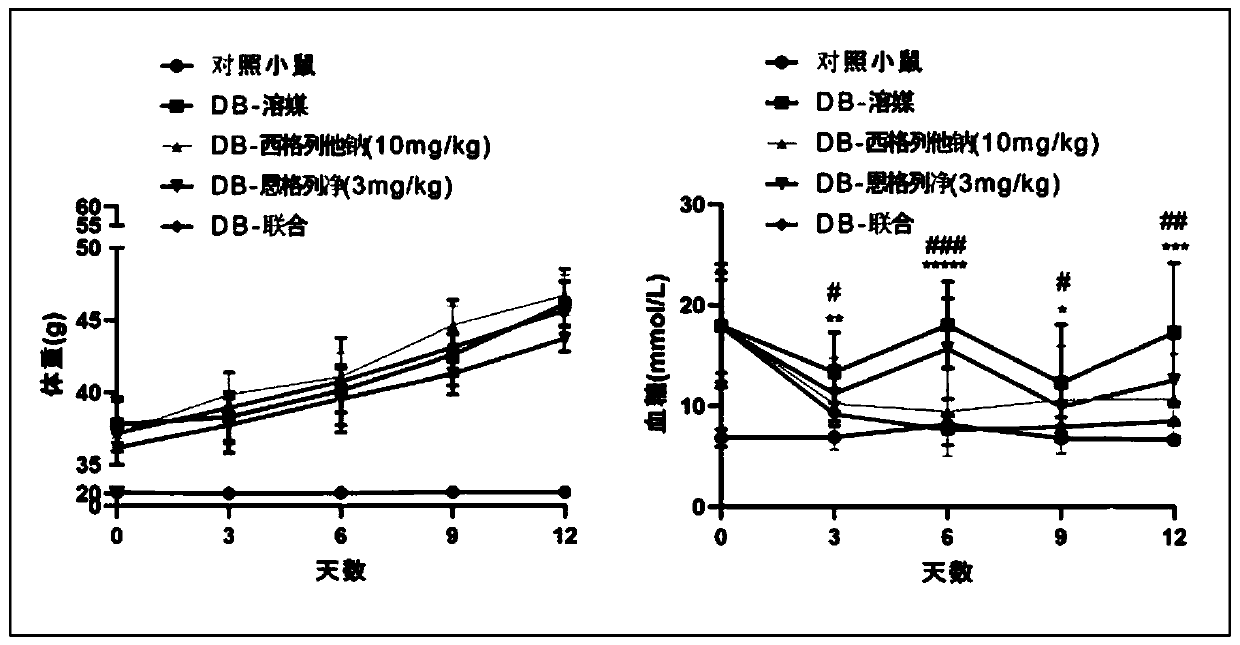
[0060] Example 1 Comparing the effect of siglitastat sodium combined with empagliflozin on body weight and blood sugar compared with single treatment
[0061] Experimental design: In diabetic mice, siglitastat sodium, empagliflozin or the combination of the two were orally administered, and the effects of single drug and drug combination on the body weight and blood sugar of the mice were investigated.
[0062] Test process: 6-week-old db / db male mice were orally administered 100 microliters of vehicle (0.1% sodium carboxymethylcellulose), 10 mg / kg siglitastat sodium, 3 mg / kg Engel Liejing or the same dose of siglitastat sodium and empaglitazine orally at the same time. Wild-type C57BLKS / Jnju mice were used as normal control mice (administered with vehicle). The dosing cycle was 14 days, and the animals were dissected on the 15th day. From the beginning of drug administration, mice were weighed every 3 days and fasting blood glucose was measured (fasting for 4-6 hours). Tes...
Embodiment 2
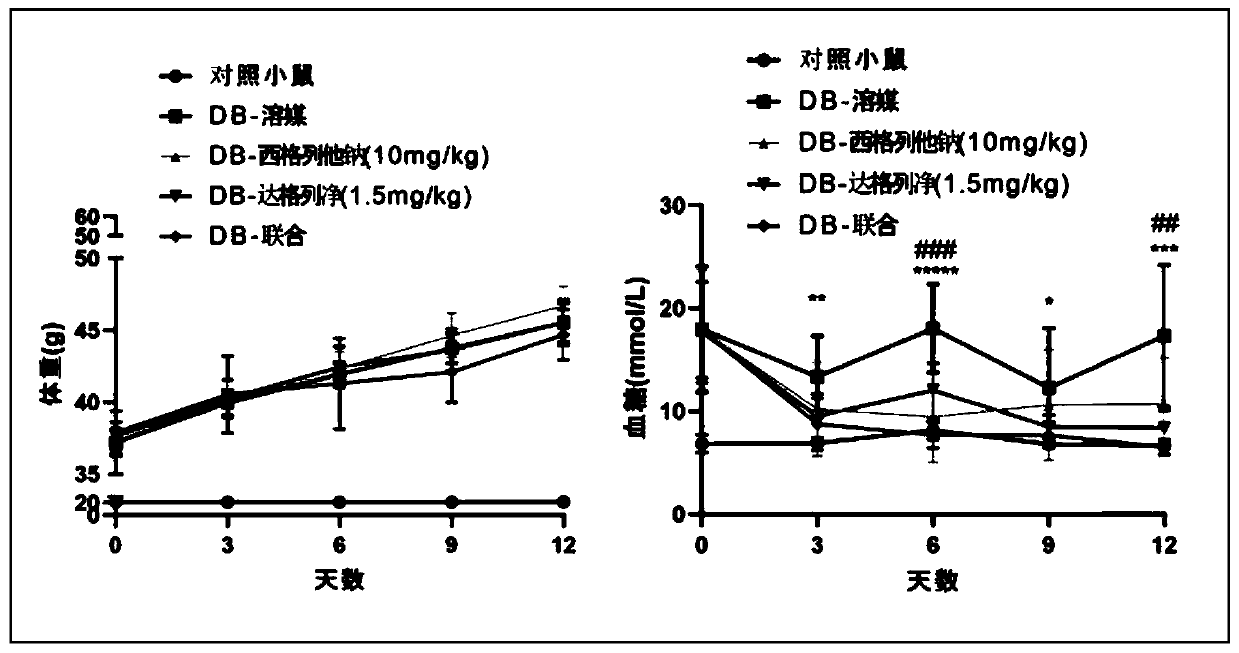
[0064] Example 2 Comparing the effect of siglitastat sodium combined with dapagliflozin on body weight and blood sugar compared with single treatment
[0065] Experimental design: In diabetic mice, siglitastat sodium, dapagliflozin or the combination of the two were orally administered, and the effects of the single drug and drug combination on the body weight and blood sugar of the mice were investigated.
[0066] Test process: 6-week-old db / db male mice were orally administered 100 microliters of vehicle (0.1% sodium carboxymethylcellulose), 10 mg / kg siglitastat sodium, 1.5 mg / kg Dagretal once a day according to their body weight Liejing or siglitastat sodium and dapaglitazine at the same dose orally at the same time. Wild-type C57BLKS / Jnju mice were used as normal control mice (administered with vehicle). The dosing cycle was 14 days, and the animals were dissected on the 15th day. From the beginning of drug administration, mice were weighed every 3 days and fasting blood...
Embodiment 3
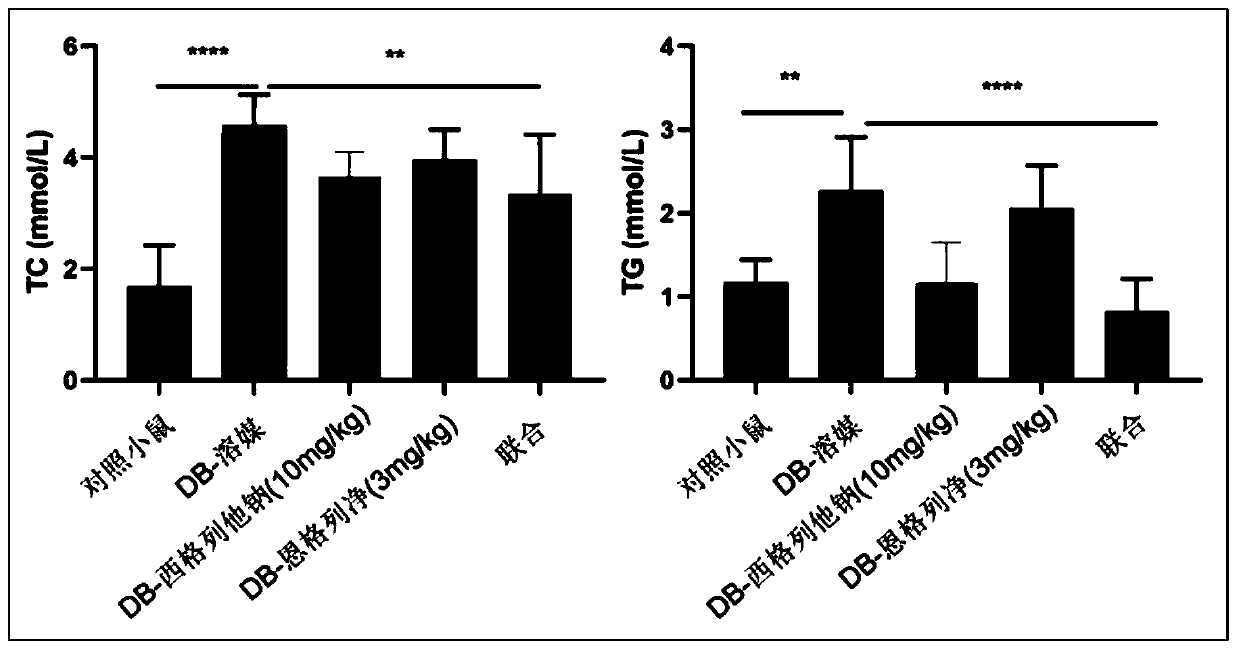
[0068] Example 3 Effect of siglitastat sodium combined with empagliflozin on blood lipids
[0069] Experimental design: In diabetic mice, siglitastat sodium, empagliflozin or the combination of the two were administered orally, and the effects of single drug and drug combination on total cholesterol (TC) and triglyceride (TG) in serum of mice were investigated.
[0070] Test process: 6-week-old db / db male mice were orally administered 100 microliters of vehicle (0.1% sodium carboxymethylcellulose), 10 mg / kg siglitastat sodium, 3 mg / kg Engel Liejing or the same dose of siglitastat sodium and empaglitazine orally at the same time. Wild-type C57BLKS / Jnju mice were used as normal control mice (administered with vehicle). The dosing cycle was 14 days, and the animals were dissected on the 15th day. Whole blood was collected, the serum was collected by centrifugation, and the TC and TG of the serum were measured by a biochemical analyzer. Test results such as image 3 shown.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com