Preparation method of potassium thiosulfate
A technology of potassium thiosulfate and potassium bisulfite, applied in the direction of thiosulfate/dithionite/polythionite, etc., which can solve the problems of long reaction time, long production cycle, and unstable finished products. To achieve the effect of shortening the reaction time, reducing equipment investment, and simple production and operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
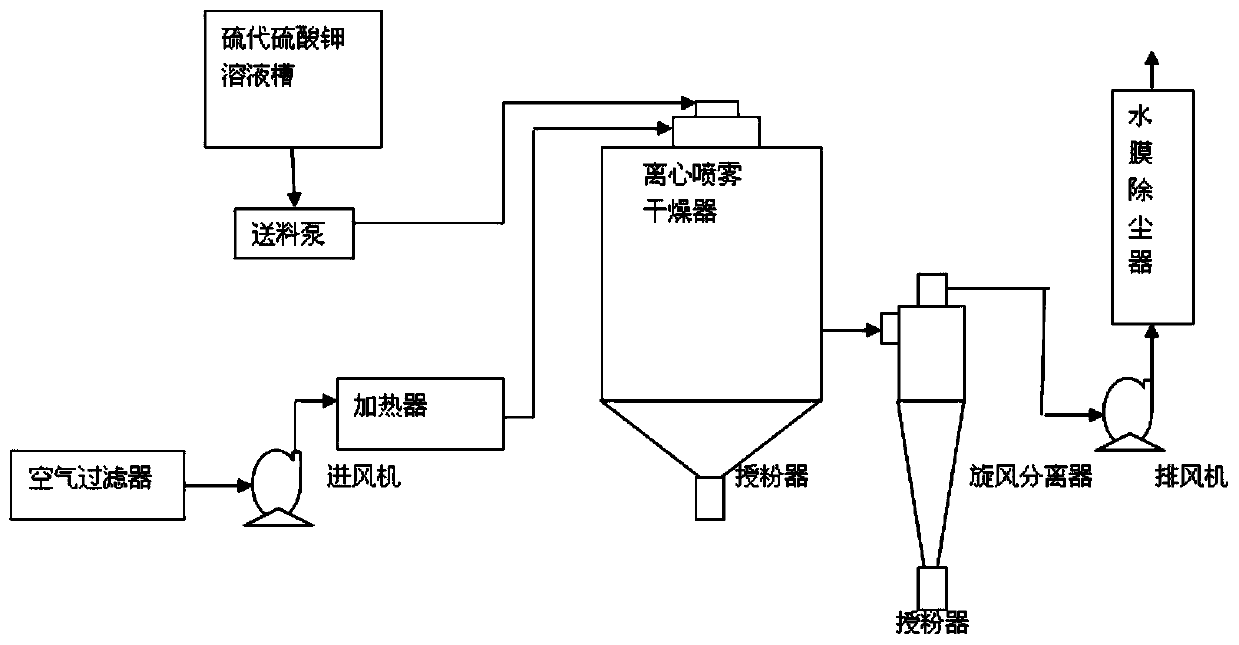
Image
Examples
Embodiment 1
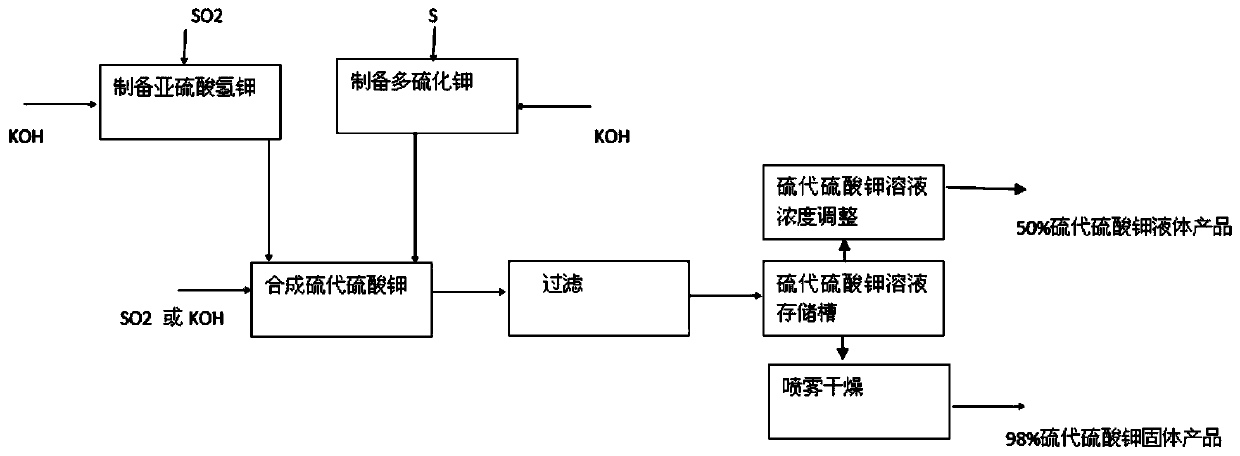
[0031] Such as figure 1 shown.
[0032] A kind of preparation method of potassium thiosulfate adopts the following steps:
[0033] 1) Preparation of potassium bisulfite solution: put 1500 kg of potassium hydroxide solution with a mass percentage concentration of 45% into the A batching tank, add water to 1500 liters, start stirring and cooling operations, and the molar ratio of potassium hydroxide to sulfur dioxide is 1:1.00 ratio, feed 812 kilograms of sulfur dioxide with a percentage content of 95% into the potassium hydroxide solution to generate a high-concentration potassium bisulfite solution, control the reaction temperature at 60°C, and the reaction completion solution is called A solution. Use; the cooling operation of this step is realized by jacket cooling water. The reaction formula KOH+SO involved in this step 2 =KHSO 3 ;
[0034] 2) Preparation of potassium polysulfide alkali solution: 1500 kg of potassium hydroxide solution with a mass percentage concentrat...
Embodiment 2
[0046] The preparation method of potassium thiosulfate as described in embodiment 1, the difference is,
[0047] In step 1), the mass percent concentration of potassium hydroxide solution is 48%, and it is put into the batching tank of A, and the molar ratio of potassium hydroxide to sulfur dioxide is 1:1.02;
[0048] In step 2), the mass percent concentration of the potassium hydroxide solution is 48%, the total feed amount of potassium hydroxide and the molar ratio of sulfur are 1:0.502, and adding mass percent content to the potassium hydroxide solution is 95% sulfur 433 kg, heat at 90°C until all the sulfur is dissolved;
[0049] In step 3), dilute potassium hydroxide solution is further added to fine-tune the pH to 6.5, which is the end point of the reaction; the reaction temperature is controlled at 90°C.
[0050] After the reaction reached the end point, the solution was sampled and analyzed for determination, and the mass percent content of potassium thiosulfate was 5...
Embodiment 3
[0052] The preparation method of potassium thiosulfate as described in embodiment 1, the difference is,
[0053] In step 1), the molar ratio of potassium hydroxide to sulfur dioxide is 1:1.04, 795 kg of sulfur dioxide with a percentage content of 99% is introduced into the potassium hydroxide solution, and the reaction temperature is controlled at 80° C.;
[0054] In step 2), the total feed amount of potassium hydroxide and the molar ratio of sulfur are 1:0.503, adding 392 kilograms of sulfur with a mass percentage content of 99% to the potassium hydroxide solution, and keeping the temperature at 100 ° C until the sulfur is completely dissolved;
[0055] In step 3), dilute potassium hydroxide solution is further added to finely adjust the pH to 8, which is the end point of the reaction; the reaction temperature is controlled at 100°C.
[0056] After the reaction reached the end point, the solution was sampled and analyzed for determination, and the mass percent content of pota...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com