Preparation method of micron hydrogel with colon-specific delivery
A hydrogel and specific technology, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems such as the greater influence on the activity of digestive enzymes, and reduce the inhibitory effect , good biocompatibility and degradability effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Functional activity, biocompatibility and effect of puerarin on protease structure.
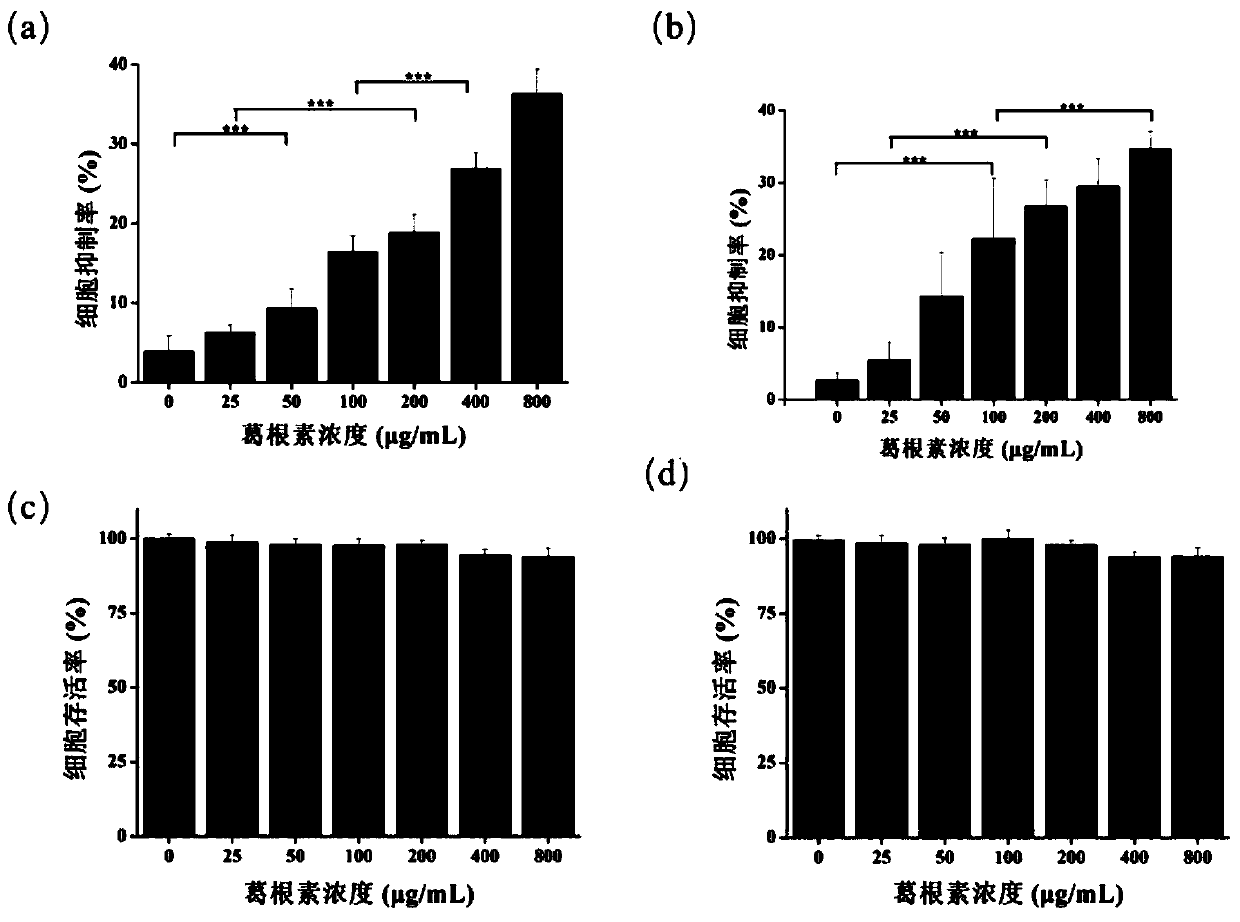
[0031] (1) Digest and count colon cancer cells (HCT116 cells) in logarithmic growth phase, adjust the initial concentration of cells to 1000 cells / well and 2000 cells / well, respectively inoculate into 96-well plates, place in 5% CO 2 After culturing in an incubator for 24 hours, the puerarin was formulated into 25, 50, 100, 200, 400, and 800 μg / mL solutions, and the puerarin solution was passed through a 0.22 μm water filter membrane. Use a pipette gun to suck out the 96-well plate cell supernatant culture solution and then add the above puerarin solution. The blank group is the blank complete medium without puerarin solution, continue to culture the cells, and use the MTT method to detect the cell viability after culturing for 72 hours and 48 hours respectively . The cell viability was obtained as figure 2 As shown in (a) and (b), the results in (a) show that the initial...
Embodiment 2
[0034] Embodiment 2: Preparation of micron hydrogel
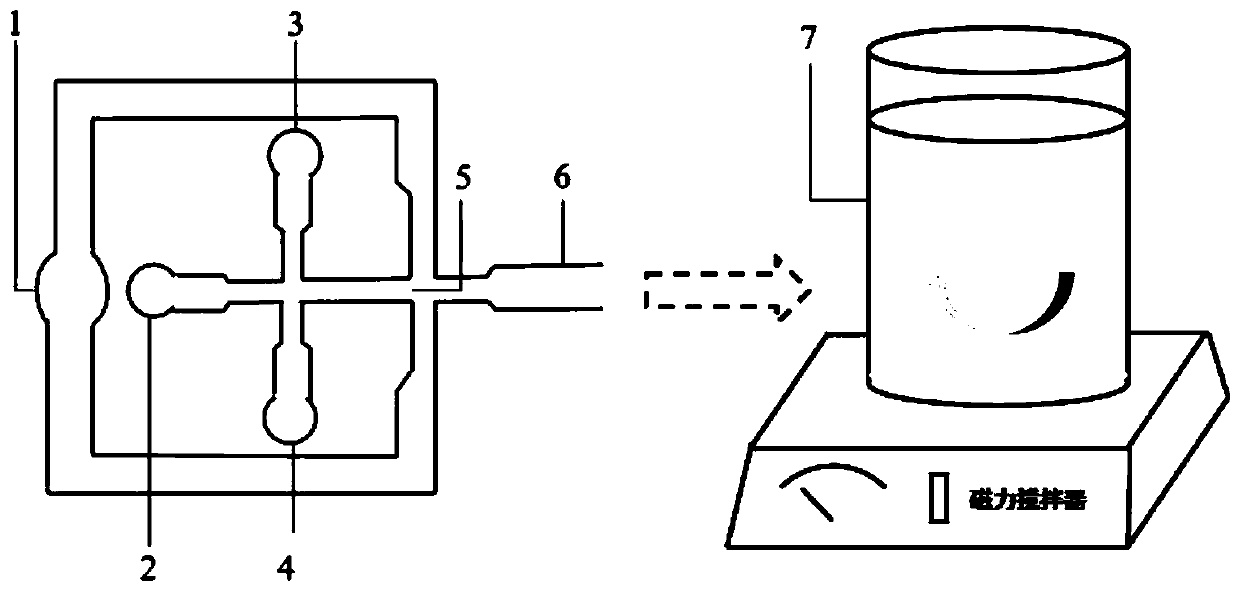
[0035](1) Puerarin was placed in sterile distilled water and stirred until completely dissolved to prepare a puerarin solution (800 μg / mL). Afterwards, the low-methoxyl pectin was added to the puerarin solution and stirred until it was fully hydrated to prepare the continuous phase, and after the low-methoxyl pectin was fully hydrated, it was left standing to remove air bubbles. Chitosan solutions were prepared by dissolving chitosan completely in acetic acid solution (1% w / v). Zinc acetate was added to the chitosan solution and stirred for 2 hours to prepare a chitosan solution containing zinc acetate (5% w / v). Sorbitan monooleate (1% w / v) was added to liquid paraffin and stirred for 2 hours, then left to stand overnight to remove air bubbles for the preparation of the dispersed phase. All experiments were performed at room temperature.
[0036] (2) Use a pressure system to inject the continuous phase and the dispersed ...
Embodiment 3
[0041] Example 3: Research on Encapsulation Efficiency and Stability of Puerarin by Hydrogel
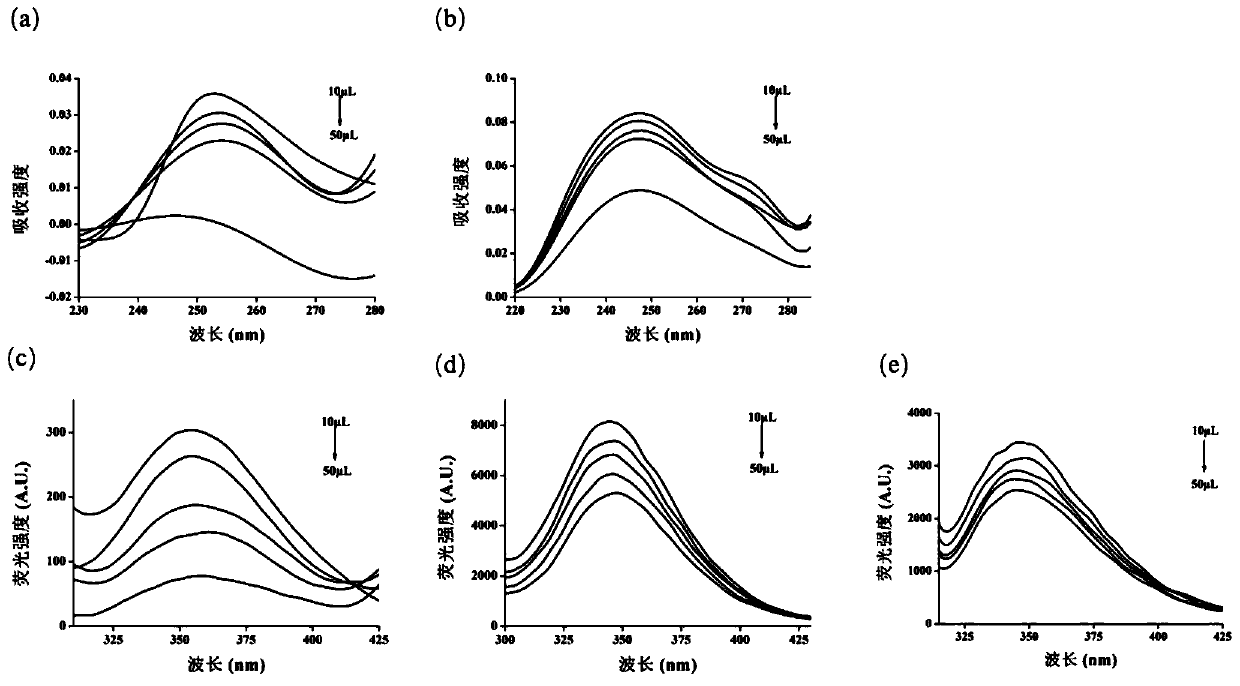
[0042] (1) use puerarin standard substance, adopt high performance liquid chromatography (HPLC) to establish standard curve, such as Image 6 Shown in (a) is the liquid chromatogram of puerarin standard substance. Pectinase and chitosanase hydrolyze the blank control group and micron hydrogel sample group loaded with puerarin, Image 6 (b) and (c) are the liquid chromatograms of the blank control group and the puerarin-loaded group, respectively. After the hydrogel was completely hydrolyzed, it was filtered through a 0.22 μm filter membrane, and the supernatant was subjected to high performance liquid chromatography to detect the content of puerarin. In this example, a Tuna C18(2) RP-HPLC system with 250x 4.6mm chromatographic column (Agilent1200, Shanghai, China). The column temperature used was 25° C., and the ratio of mobile phase methanol to water was 1:3. The detection wav...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com