Application of h.hathewayi or taurine as a medicine for preventing and treating intracranial aneurysm formation and rupture
A technology of intracranial aneurysm and taurine, which is applied in the field of medicine, can solve the problems of bleeding risk and complications, reduce the formation and rupture of intracranial aneurysm, promote the increase, inhibit the activation of matrix metalloproteinase and the blood vessel wall Effects of extracellular matrix degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
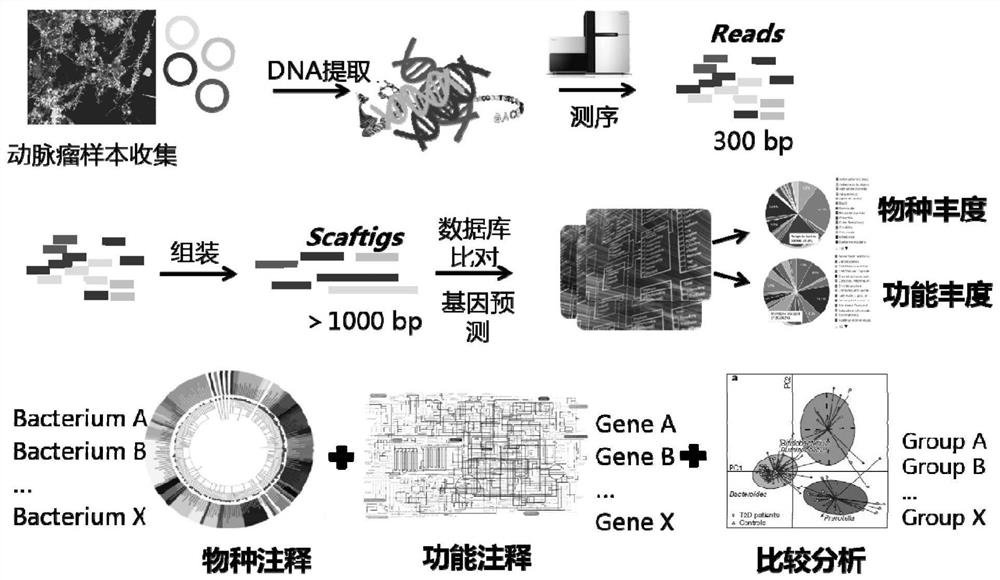
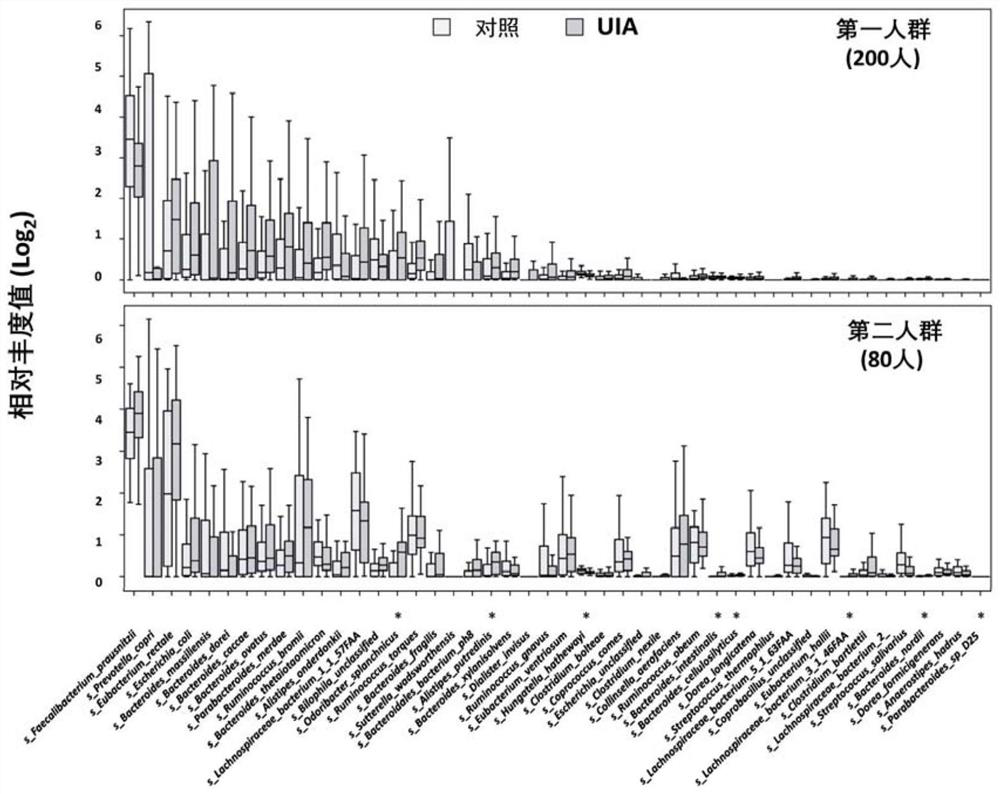
[0066] Example 1: Changes in the level of gut microbial species in patients with intracranial aneurysms compared to controls revealed by metagenomics sequencing
[0067] 1. Materials and Instruments
[0068] In this example, the Illumina HiSeq X Ten sequencing platform was used to sequence the collected human intestinal microbial DNA samples, and then the sequencing data were subjected to biological species annotation, species quantification and related bioinformatics analysis.
[0069] 2. Method
[0070] 2.1 From 2016 to 2018, 100 patients with non-ruptured intracranial aneurysms and 100 matched control populations (the first population) were enrolled in Beijing Tiantan Hospital and Hebei Cangzhou Central Hospital, and their stool samples and Plasma samples. In this study, from 2017 to 2018, 40 patients with non-ruptured intracranial aneurysms and matched control groups with corresponding clinical parameters were enrolled in Beijing Tiantan Hospital, Chinese People's Libera...
Embodiment 2
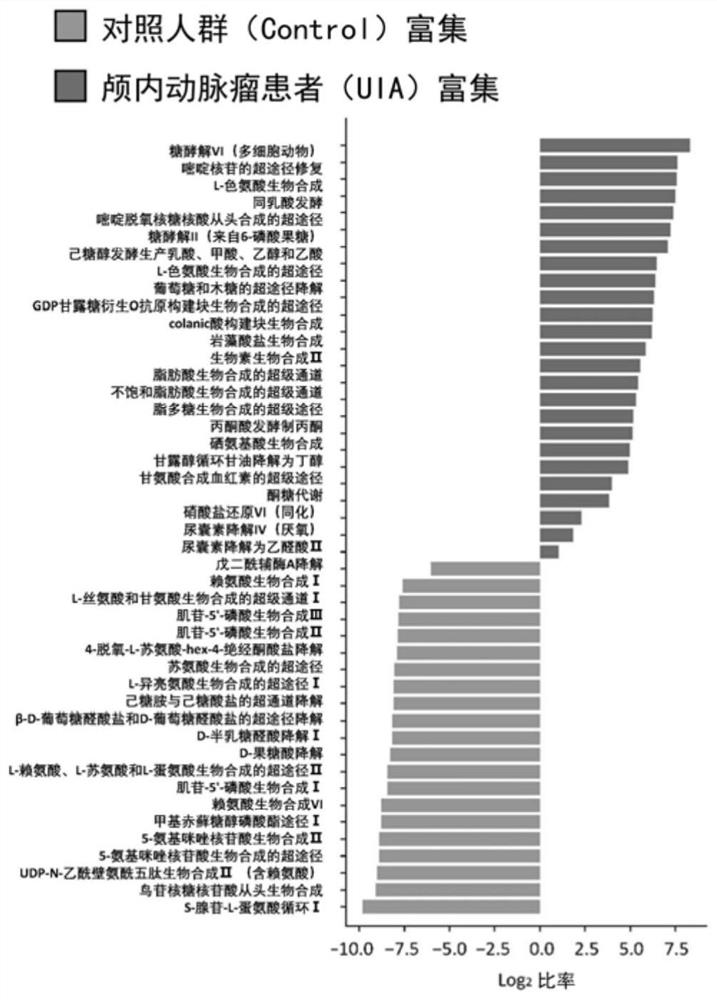
[0083] Example 2: Exploring the functional changes of gut microbes and their association with plasma metabolites in patients with intracranial aneurysm through population gut metagenomics and plasma metabolomics
[0084] 1. Materials and Instruments
[0085] In this example, the UPLC-MS / MS system was used to detect the metabolites (amino acids and free fatty acids) in the plasma of the population by targeted metabolomics.
[0086] 123 standard amino metabolites were purchased from Sigma-Aldrich (Shanghai) Trading Co., Ltd. (Sigma-Aldrich, USA) and Beijing Bailingwei Technology Co., Ltd. (J&K, China; TRC, Canada). Boronic acid, N-ethylmaleimide (NEM), 4-tert-butylthiophenol (tBBT), dimethylsulfoxide (DMSO), 5-aminoisoquinoline (5AIQ), N,N' -Disuccinimidyl carbonate (DSC), ascorbic acid (Vc), ethylenediaminetetraacetic acid (EDTA) and tris(2-carboxyethyl)phosphine (TCEP) were purchased from Sigma-Aldrich (Shanghai) Trading Ltd. (Sigma-Aldrich, USA), analytically pure dipotassi...
Embodiment 3
[0100] Example 3: Feces from patients with intracranial aneurysms are more likely to cause the formation and rupture of intracranial aneurysms in mice than feces from normal people through fecal transplantation experiments
[0101] 1. Materials and Instruments
[0102] Table-3 Materials and instruments required for preparation and evaluation of mouse intracranial aneurysm model
[0103]
[0104]
[0105] 2. Method
[0106] 2.1 Donor feces acquisition
[0107] From 2017 to 2018, 2 cases of feces from patients with intracranial aneurysms and 2 cases of normal controls were collected in the General Hospital of the Chinese People's Liberation Army and Tsinghua University Hospital for mouse fecal transplantation experiments. Donor clinical information is as follows:
[0108] Table 4 Donor clinical characteristics
[0109]
[0110] 2.2 Experimental animals
[0111] The experimental design complied with the implementation rules for the management of medical experimental...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com