Recombinant fibronectin mutant and application thereof
A fibronectin and mutant technology, applied in the field of biology, can solve the problems of protein misfolding and aggregation, slowness, and inability to obtain soluble protein, and achieve the effect of improving cell adhesion activity, promoting cell proliferation activity, and convenient purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 recombinant fibronectin carrier construction
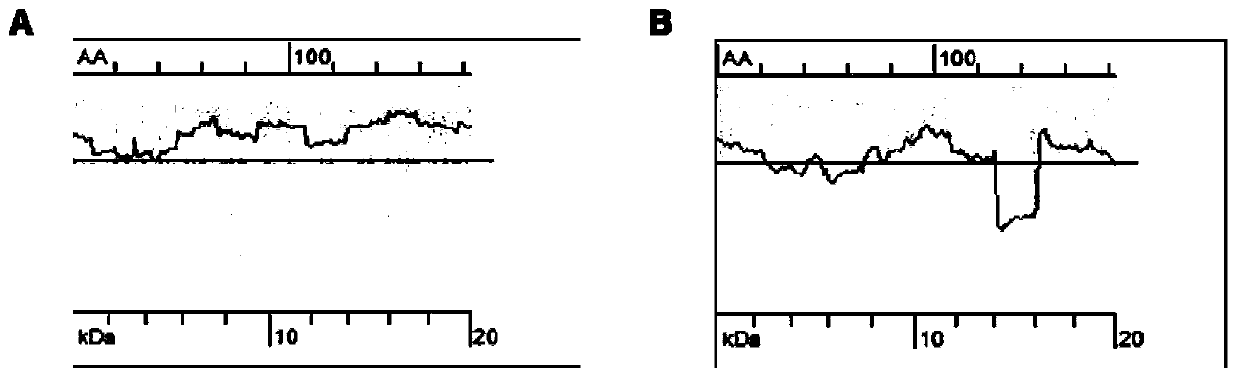
[0037] We selected the functional domains of fibronectin to promote cell proliferation and adhesion through biological macromolecule simulation, and designed new fibronectin mutants. The nucleotide sequences of the fibronectin mutants were optimized according to the codon bias of E. coli and by translation pause theory. And entrusted Suzhou Synbio Biotechnology Co., Ltd. to synthesize the fibronectin mutant DNA from the whole gene.
[0038] The nucleotide sequence of unoptimized recombinant fibronectin is as follows:
[0039] ACGGGCATCGACTTCAGCGATATCACCGCGAACAGCTTCACCGTTCACTGGATCGCGCCACGTGCGACGATCACCGGCTATCGCATCCGCCATCACCCGGAACACTTTAGCGGTCGTCCACGCGAAGATCGCGTTCCGCATAGCCGCAATAGCATCACGCTGACCAATCTGACCCCGGGCACCGAATATGTTGTGAGCATCGTGGCGCTGAACGGCCGCGAAGAAAGCCCACTGCTGATTGGCCAGCAGAGCACCGTGAGTGATGGTGGCGGTGGCAGCAATATTGATCGCCCGAAAGGTCTGGCCTTCACGGATGTGGACGTGGACAGCATCAAAATCGCGTGGGAAAGCCCACAAGGCCAAGTTAGCCGCTACCGCGTGACCTATAGCA...
Embodiment 2
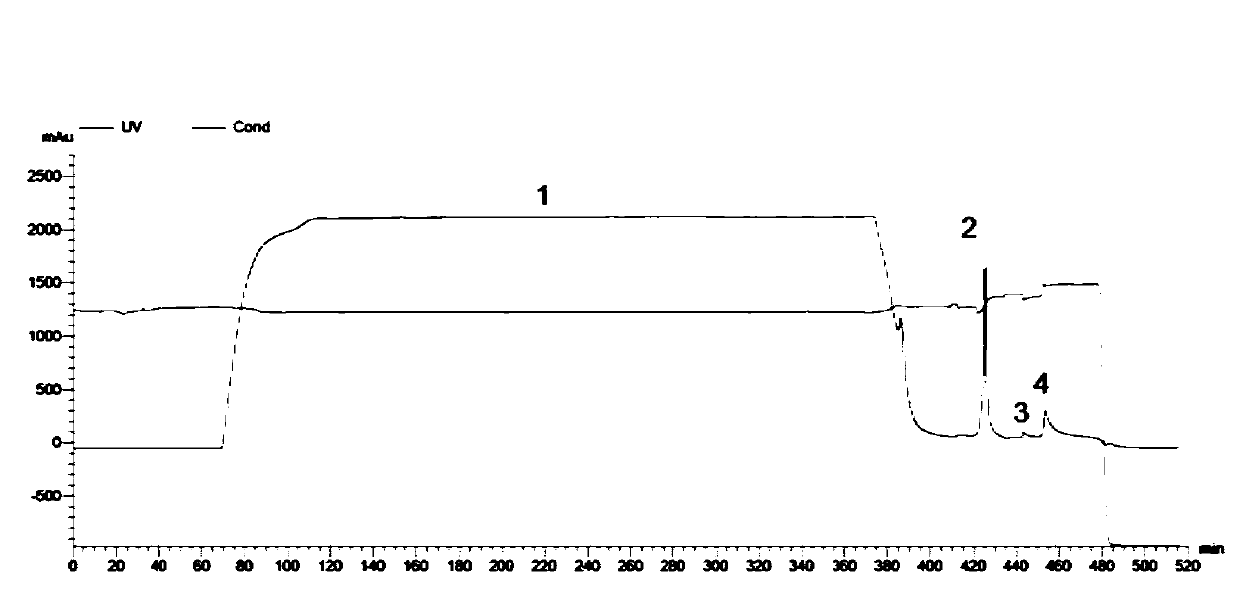
[0046] Expression and purification of embodiment 2 recombinant fibronectin mutants
[0047] (1) Preparation of fibronectin expression strain:
[0048] The preparation process of the fibronectin expression bacterial strain is:
[0049] ① Preparation of Escherichia coli BL21(DE3) competent cells: For the preparation process, please refer to the third edition of "Molecular Cloning Experiment Guide"; [US] J. Sambrook, translated by Huang Peitang.
[0050] ②Transform the expression vector pET-28a-Fibronectin into Escherichia coli BL21(DE3) competent cells: see the third edition of "Molecular Cloning Experiment Guide" for details on the transformation process; [US] J. Sambrook, translated by Huang Peitang.
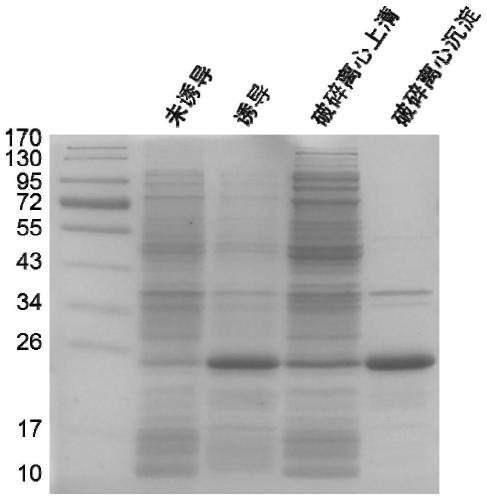
[0051] (2) Induced expression and solubility analysis of fibronectin mutants
[0052] The specific operation process is as follows:
[0053] The expression strain pET-28a-Fibronectin obtained in step (1) was inoculated into 10 mL of LB medium containing 50 μg / mL kanamycin con...
Embodiment 3
[0065] Example 3 Determination of the Proliferative Activity of Recombinant Fibronectin Mutants
[0066] The specific measurement process is:
[0067] (1) BALB / c 3T3 cells were seeded in 96-well cell culture plates (5000 cells / well), 37°C, 5% CO 2 Cultivate in a cell incubator for 24 hours;
[0068] (2) Replace with DMEM serum-free medium and continue culturing for 12 hours;
[0069] (3) Add recombinant fibronectin mutants (soluble expression purification fraction, inclusion body expression purification fraction) and PBS (negative control group) respectively, and continue culturing for 48-72 hours;
[0070] (4) Add 10 μL CCK-8 reagent to each well, 37°C, 5% CO 2 Take it out after incubating in the cell incubator for 2 hours;
[0071] (5) Read the absorbance values of the 96-well plate at 450nm and 630nm with a microplate reader, take 630nm as the reference wavelength, measure the absorbance at 450nm, and record the measurement results.
[0072] Relative cell proliferati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com