Method for detecting kasugamycin by high performance liquid chromatography
A high-performance liquid chromatography and kasugamycin technology, which is applied in the field of high-performance liquid chromatography for detecting kasugamycin, can solve the problems of inconvenient analysis work, damaged chromatographic column, complicated operation and the like, and achieves simple preparation, rapid and accurate detection and detection. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
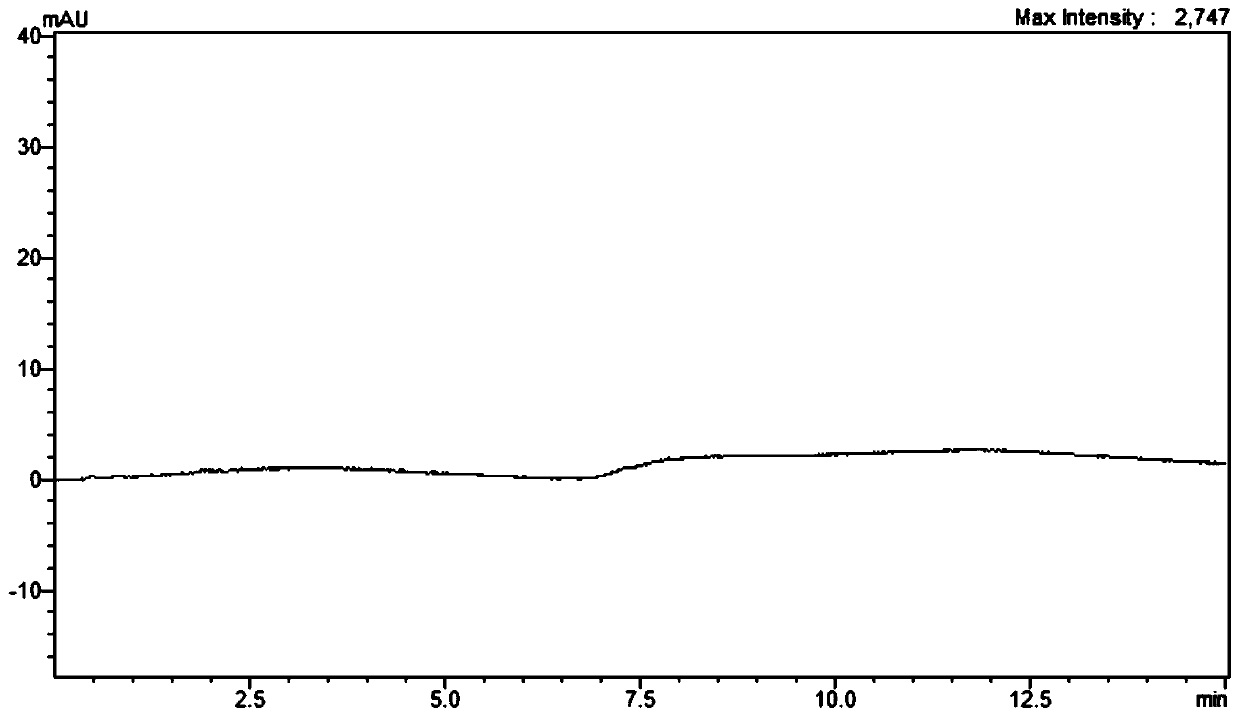
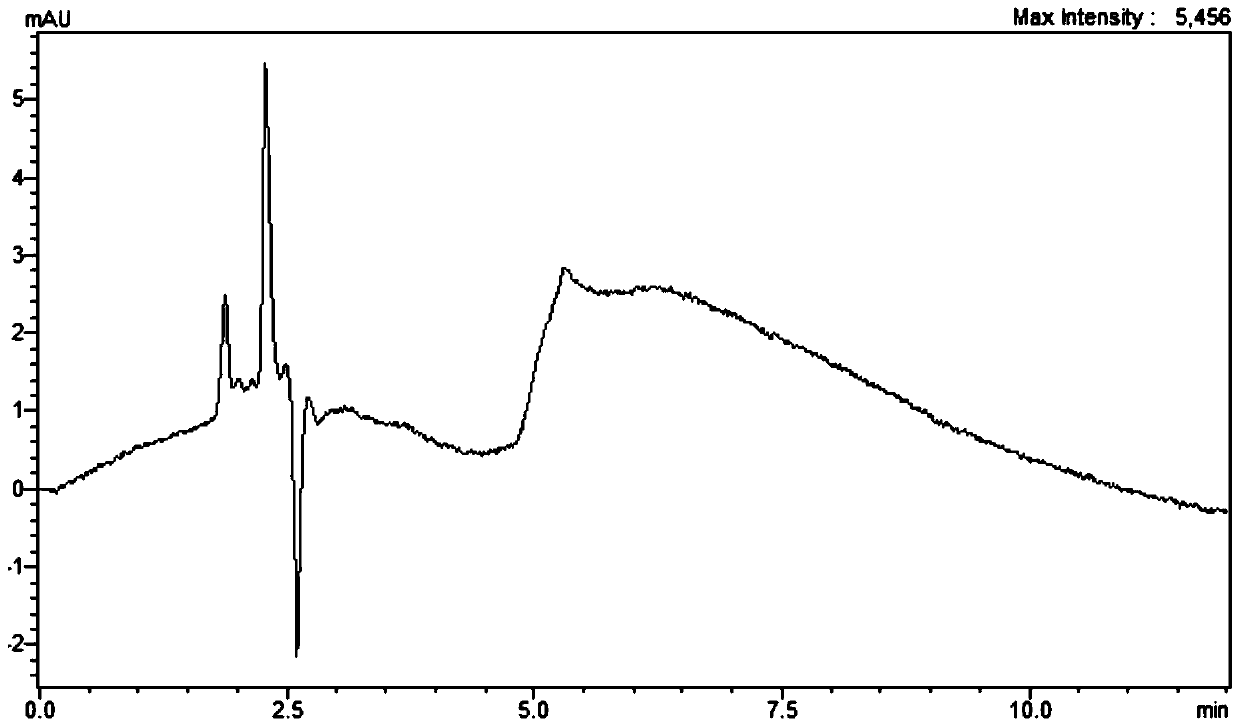
[0033] Chromatographic conditions:
[0034] Chromatographic column: Agela Venusil HILIC (Agela, 4.6mm×150mm, 5μm); mobile phase: acetonitrile: ammonium formate-formic acid buffer solution = 70:30 (volume ratio); elution method: isocratic elution; flow rate: 1.0mL / min; detection wavelength: 220-245nm; column temperature: 40°C; injection volume: 10μL; detector adopts diode array detector, elution time: 8min.
[0035] The specific operation steps are:
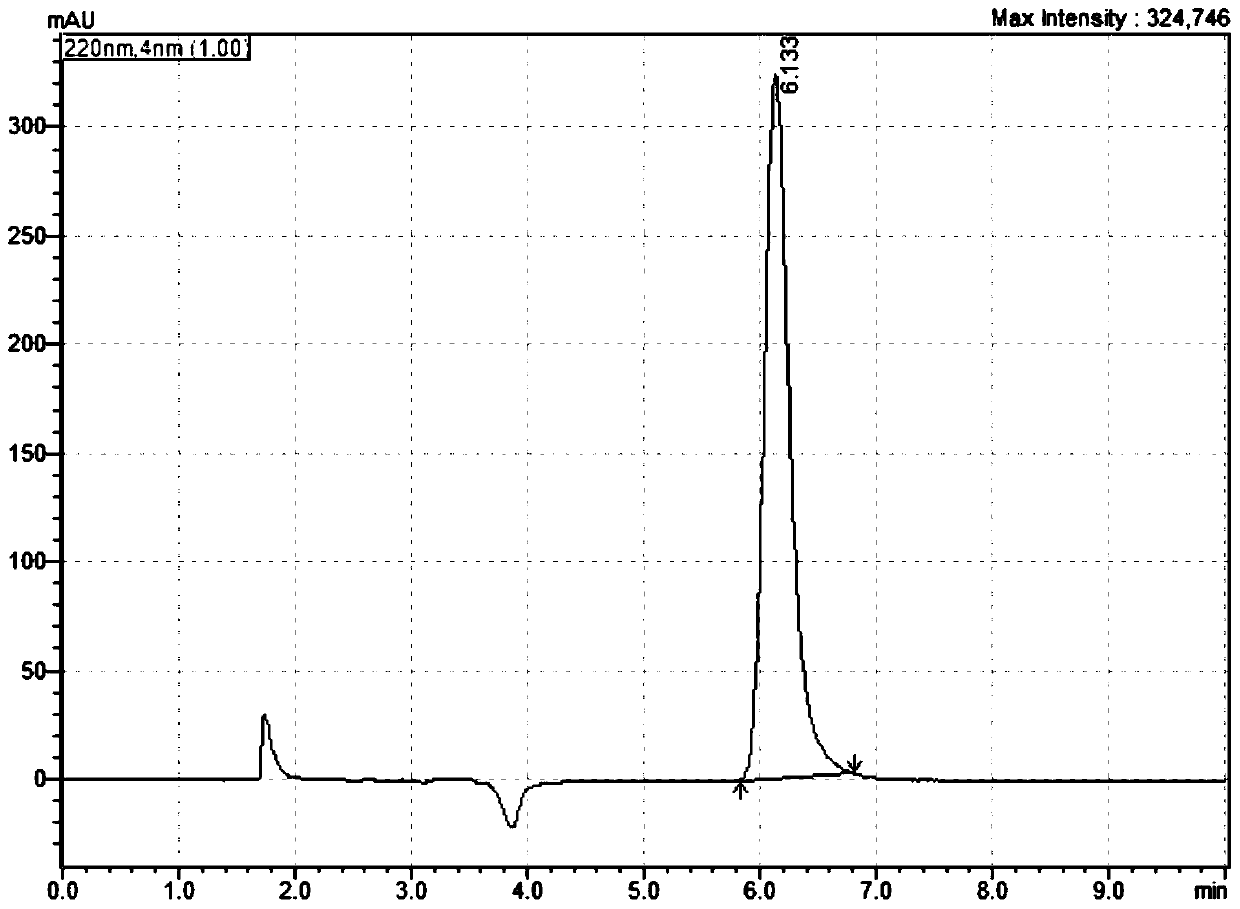
[0036] (1) Weigh 0.0629g of kasugamycin standard substance, place it in a 100ml volumetric flask, add 90ml of water phase, dissolve the standard substance by ultrasonic vibration, cool to water temperature, dilute with water phase to the mark, shake well, and obtain kasugamycin standard The product solution is ready for use;
[0037] (2) Weigh 0.0649g of the kasugamycin sample to be tested, place it in a 100ml volumetric flask, add 90ml of the water phase, dissolve the sample to be tested by ultrasonic vibration, cool to water t...
Embodiment 2
[0052] According to the detection method of embodiment 1, the composition of mobile phase has been studied, and the relation of concrete mobile phase composition and peak time is as follows table 2:
[0053] Table 2-Relationship between mobile phase composition and peak elution time
[0054]
[0055]
[0056] According to the detection spectrum, it is found that the detection wavelength range of the present invention is 220-245nm, and has the following characteristics. With the red shift of the wavelength, the peak area of kasugamycin has a downward trend, and with the blue shift of the wavelength, the baseline will become worse.
Embodiment 3
[0058] In order to verify the feasibility and accuracy of the detection method of the present invention, now take Example 1 as an example to carry out the following verification test:
[0059] (1) The precision and accuracy of the sample
[0060] Chromatographic conditions:
[0061] Chromatographic column: Agela Venusil HILIC (Agela, 4.6mm×150mm, 5μm); mobile phase: acetonitrile: ammonium formate-formic acid buffer solution = 70:30 (volume ratio); flow rate: 1.0mL / min; detection wavelength: 220 -245nm; Column temperature: 40°C; Injection volume: 10μL; The detector uses a diode array detector;
[0062] Under the above-mentioned stable chromatographic conditions, the kasugamycin sample to be tested in Example 1 with the same content was tested repeatedly for 6 times to test the precision of the method. The precision experiment result of the present invention is as follows table 3:
[0063] Table 3 - Results of precision experiments
[0064]
[0065] From the experimental ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com