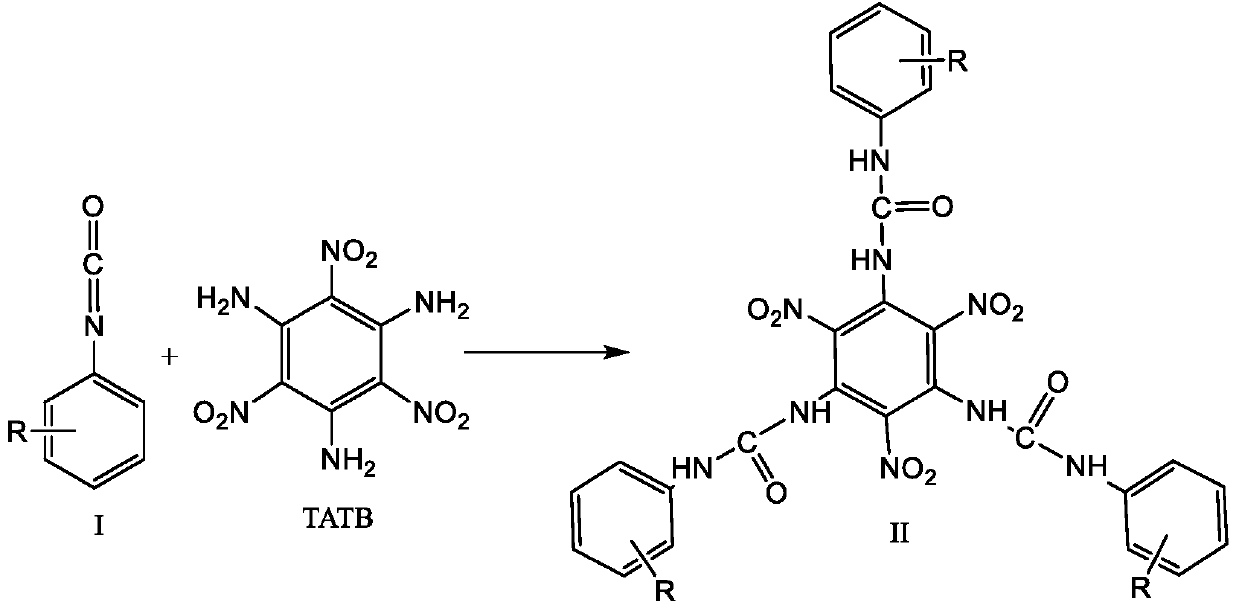
Method of purifying TATB
A technology for separation, purification and substitution of urea, applied in chemical instruments and methods, preparation of organic compounds, purification/separation of amino compounds, etc., can solve problems such as low solubility, complicated operation and difficulty, and achieve mild purification conditions and good separation effect. , safe to use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
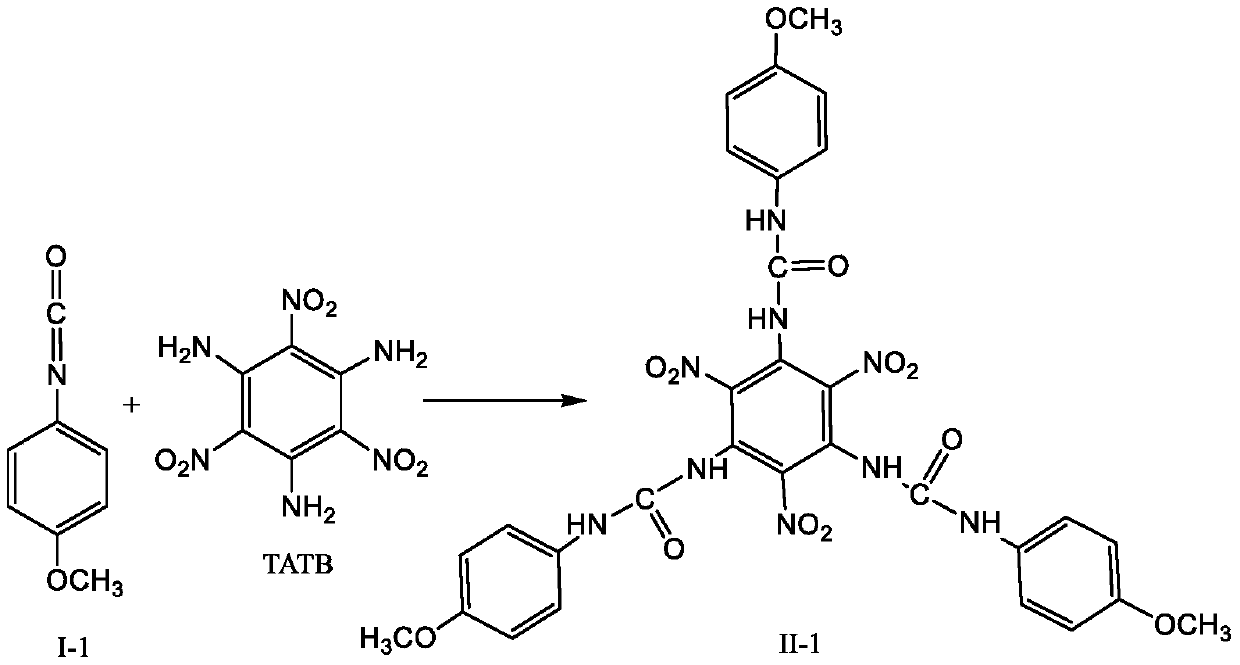
[0021] 1. Under the protection of anhydrous, oxygen and nitrogen, add 2.58g (10mmol) TATB and 4.47g (30mmol) 4-methoxyphenylisocyanate shown in formula I-1 into 100mL dry dimethyl sulfoxide , stirred and reacted at 50°C for 30 minutes, filtered to remove insoluble substances after the reaction, and then distilled off unreacted 4-methoxyphenyl isocyanate and most of dimethyl sulfoxide under reduced pressure, and added ethyl acetate to the concentrated solution , the substituted urea was precipitated from the solvent, and then cooled in an ice-water bath to completely precipitate the substituted urea, then suction filtered and washed with ether to obtain a pure substituted urea represented by formula II-1.
[0022]
[0023] 2. Add the substituted urea shown in formula II-1 into 50 mL of a mixed solvent with a volume ratio of dimethyl sulfoxide and deionized water of 1:0.2, add concentrated hydrochloric acid to adjust the pH to 1, and then heat the reaction at 80°C for 6 hours...
Embodiment 2
[0025] 1. Under the protection of anhydrous, oxygen and nitrogen, add 2.58g (10mmol) TATB and 3.99g (30mmol) p-toluene isocyanate represented by formula I-2 into 100mL dry dimethyl sulfoxide, at 50°C Stir the reaction for 30 minutes, filter to remove insoluble matter after the reaction, then distill under reduced pressure to remove unreacted p-toluene isocyanate and most of dimethyl sulfoxide, add ethyl acetate to the concentrated solution to precipitate the substituted urea from the solvent, and then After cooling in an ice-water bath to completely precipitate the substituted urea, filter it with suction and wash with ethyl acetate to obtain the pure substituted urea represented by formula II-2.
[0026]
[0027] 2. Add the substituted urea shown in formula II-2 into 60 mL of a mixed solvent with a volume ratio of dimethyl sulfoxide and deionized water of 1:0.3, and add NaOH to adjust the pH to 14, and then heat and react at 60°C for 6 hours , filtered after the reaction, ...
Embodiment 3
[0029] 1. Under anhydrous, oxygen-free and nitrogen protection, add 2.58g (10mmol) TATB and 5.61g (30mmol) 4-(trifluoromethyl)phenylisocyanate shown in formula I-3 to 100mL dry dimethyl In sulfoxide, stir and react at 50°C for 30 minutes, filter to remove insoluble matter after the reaction, then distill under reduced pressure to remove unreacted 4-(trifluoromethyl)phenyl isocyanate and most of dimethyl sulfoxide, concentrate Add deionized water to the solution to precipitate the substituted urea from the solvent, then cool in an ice-water bath to completely precipitate the substituted urea, filter it with suction, and wash with ether to obtain the pure substituted urea shown in formula II-3.
[0030]
[0031] 2. Add the substituted urea shown in formula II-3 into 55 mL of a mixed solvent with a volume ratio of dimethyl sulfoxide and deionized water of 1:0.25, and add NaOH to adjust the pH to 14, then heat and react at 65°C for 5 hours , filtered after the reaction, and was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com