Construction method and application of recombinant drug-resistant BCG strain
A construction method and technology of drug-resistant bacteria, applied in the field of genetic engineering, can solve problems such as inability to cause immunity and microecological protection, and achieve the effects of reducing the risk of re-infection, accelerating death, and protecting patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation method of embodiment 1 drug-resistant BCG bacterial strain
[0027] 1. Preparation of 7H9 and 7H11 medium
[0028] 1. 7H9 liquid medium: 4.7g 7H9 powder, 2mL glycerol, 900mL ddH 2 O, high temperature and high pressure sterilization, add 100mL OADC to use after cooling;
[0029] 2. 7H11 solid medium: 19g 7H11 powder, 5mL glycerol, 900mL ddH 2 O, high temperature and high pressure sterilization, add 100mL OADC to use after cooling;
[0030] 2. Screening for spontaneous drug-resistant BCG
[0031] 1. According to the MIC of M.bovis BCG (hereinafter referred to as wild-type BCG bacteria, provided by Johns Hopkins University School of Medicine) to streptomycin is 0.25 μg / mL, prepare 7H11 plate containing streptomycin (final concentration is 0.25* MIC-8*MIC) and 7H11 blank board. After diluting the M.bovis BCG grown to the logarithmic phase to an appropriate concentration, pipette 500 μL of the bacterial solution and spread it evenly on the plate. Place ...
Embodiment 2
[0044] Example 2 Construction of recombinant drug-resistant BCG strains
[0045] 1. Preparation of recombinant plasmids containing Ag85b gene and Rv2628 gene fragments.
[0046] 1. PCR product recovery and digestion
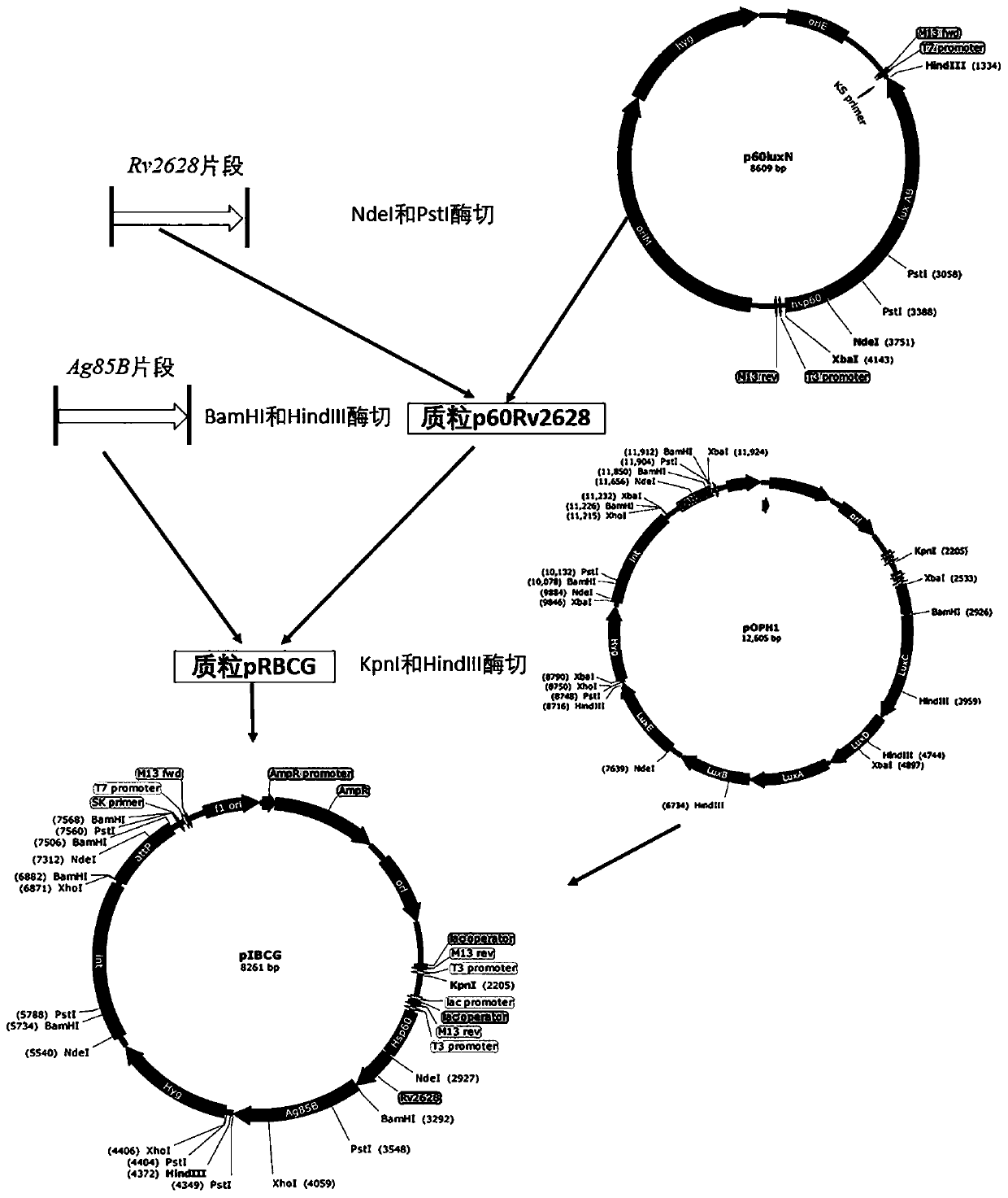
[0047] The plasmid construction process is as follows figure 1 . Using the genomic DNA of Mtb H37Rv (provided by Guangzhou Chest Hospital) as a template, the Ag85b gene was amplified by PCR (primer pair——F:
[0048] 5'-CGGGATCCATGAGACGACTTTGACGCCCGAA-3', R: 5'- CCCCTGCAGGGATCCTTAGACCGCAACGGCAATCT-3') and Rv2628 gene (primer pair - F:
[0049] 5'-GGAATTCCATATGATGTCCACGCAACGACCGA-3', R: 5'-CCCCTGCAGGGATCCTTAGACCGCAACGGCAATCT-3') fragment, the PCR product was recovered after 0.8% agarose gel electrophoresis and identified by sequencing.
[0050] The recovered Rv2628 fragment after identification and the starting plasmid p60LuxN (constructed by our laboratory, see the figure for the plasmid map, and refer to the related content of CN 201810359440.4 for the specif...
Embodiment 3
[0065] Plasmid stability detection of embodiment 3 recombinant drug-resistant BCG
[0066] The obtained recombinant drug-resistant BCG bacterial solution was diluted to an appropriate concentration, cultured in 7H9 medium containing hygromycin, and continuously subcultured five times. The bacterial solutions passed down to the fourth and fifth generations were respectively serially diluted, and the bacterial solutions were spread on 7H11 plates containing hygromycin, and cultured in an incubator for four weeks. Pick a single colony for amplification and culture and extract its DNA, use primers to amplify and identify Mark-F (5'-CGATGTGGTCGGATAGGCA-3') and Mark-R (5'-ACTCACCTGCGGTTTATCTGC-3'), and amplify 0.6 The kb fragment includes the Ag85B gene and the Rv2628 gene.
[0067] Randomly pick colonies from 5 consecutive subcultures, and use the above primer pair (Mark-F and Mark-R) to carry out colony PCR. The results are as follows: figure 2 ;Depend on figure 2 It can be o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com