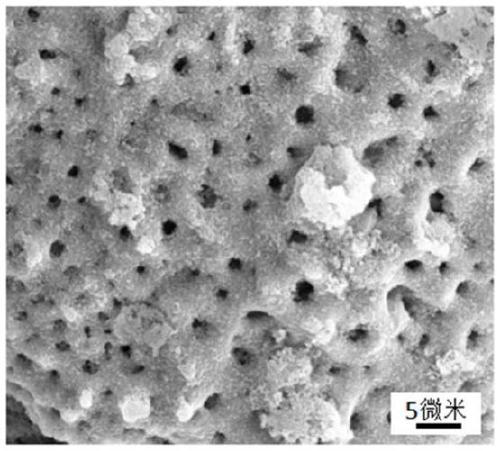
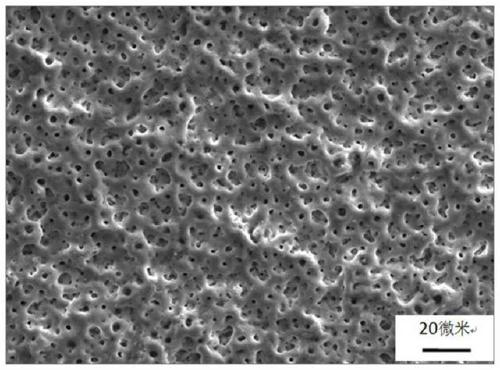
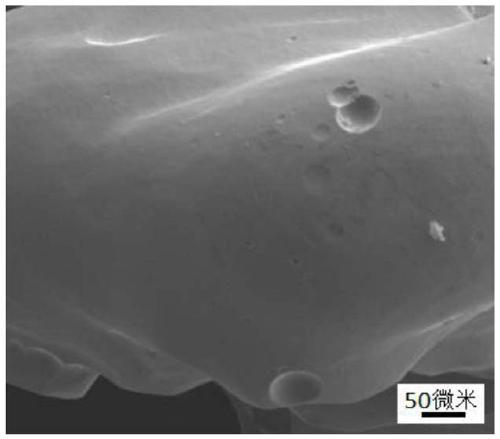
Preparation method of bioactive porous tantalum implant
A bioactive, porous tantalum technology, used in tissue regeneration, prostheses, coatings, etc., can solve the problems of suboptimal surface bioactive modification, no promotion of new bone formation, and inability to achieve modification, etc. Osteoinduction and bone ingrowth, improved distribution uniformity and stability, reasonable proportions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation steps of bioactive functional porous tantalum implant:
[0049] The three-dimensional porous tantalum implant material prepared by vapor deposition method is used as the modification object of Example 1. The three-dimensional porous tantalum implant material is an intervertebral fusion device with a height of 8 mm. The intervertebral fusion device has a porous structure. The intervertebral fusion The device has a central hole, the diameter of the inscribed circle of the central hole is 8mm, the diameter of the circumscribed circle is 20mm, and the angle between the upper and lower end surfaces is 8°. Carry out pickling treatment on the intervertebral fusion cage, the pickling solution used includes hydrofluoric acid, nitric acid and water; the concentration of hydrofluoric acid is 40%, the concentration of nitric acid is 68%, the volume ratio of hydrofluoric acid, nitric acid and water It is 5:8:87; pickling time is 3 minutes. The pickled intervertebral cag...
Embodiment 2
[0051] Preparation steps of bioactive functional porous tantalum implant:
[0052] The three-dimensional porous tantalum implant material prepared by powder metallurgy is used as the modified object of Example 2. The three-dimensional porous tantalum implant material is an artificial vertebral body with a height of 50 mm. The artificial vertebral body is a porous structure. The artificial vertebral body has a The central hole, the diameter of the inscribed circle of the central hole is 6mm, the diameter of the circumscribed circle is 20mm, and the angle between the upper and lower end faces is 8°. Carry out pickling treatment to artificial vertebral body, the pickling solution that adopts comprises hydrofluoric acid, nitric acid and water; The concentration of hydrofluoric acid is 40%, the concentration of nitric acid is 68%, the volume ratio of hydrofluoric acid, nitric acid, water is 2:8:90; pickling time is 2 minutes. The artificial vertebral body after pickling was ultras...
Embodiment 3
[0054] Preparation steps of bioactive functional porous tantalum implant:
[0055] The three-dimensional porous tantalum implant material prepared by electron beam melting technology is used as the modification object of embodiment 3. The three-dimensional porous tantalum implant material is an acetabular cup, and the surface structure of the acetabular cup is a porous structure, and the spherical outer diameter of the acetabular cup is 50mm, the acetabular height is 36mm. The acetabular cup is pickled, and the pickling solution used includes hydrofluoric acid, nitric acid and water; the concentration of hydrofluoric acid is 40%, the concentration of nitric acid is 68%, and the volume ratio of hydrofluoric acid, nitric acid and water is 5:10:85; pickling time is 5 minutes. The acid-washed acetabular cup was ultrasonically cleaned with acetone and deionized water for 30 minutes in sequence. After ultrasonic cleaning, the acetabular cup was subjected to micro-arc oxidation trea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| compressive strength | aaaaa | aaaaa |
| elastic modulus | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com