Clostridium perfringens surface glycans and uses thereof
A technology of Clostridium perfringens and polysaccharides, applied in antibacterial drugs, sugar derivatives, sugar derivatives, etc., can solve the problems of high cost and low market value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Clostridium strains were incubated at 37°C under anaerobic conditions supplemented with 5% hydrogen, 5% CO 2 , 90% N 2 ) Whitley DG250 anaerobic workstation (Don Whitley Scientific, Frederick, MD), and cultured in non-agitated PGY broth (3% peptone #3, 2% glucose, 1% yeast extract, 0.1% thioethanol NaCl) or on PGY agar (PGY broth containing 1.5% agar). Table 1 lists Clostridium perfringens strains and their isolates and derivatives.
[0065] Table 1. Clostridium perfringens strains.
[0066]
[0067]
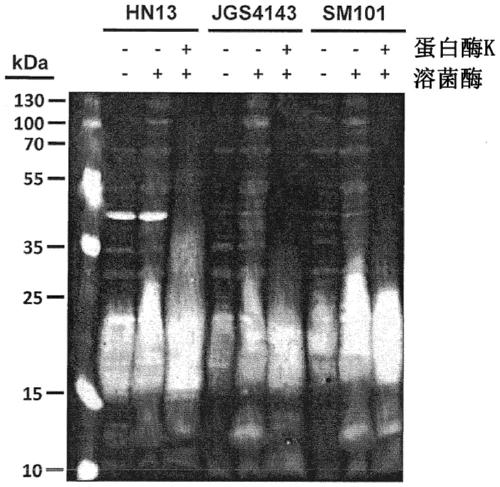
[0068] Whole cell lysates of Clostridium perfringens HN13, JGS4143 and SM101 were generated for SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis as follows: Strains were stocked from -80°C Streak onto PGY agar plates (with antibiotics as appropriate) and grow overnight. For each strain, a single colony was used to inoculate 10 ml of PGY broth, allowed to grow for 6 h, harvested by centrifugation (13 000 × g, 10 min), washed with phosph...
Embodiment 2
[0073] figure 2 The cpe2071 glycosyltransferase mutants from HN13, four different glycosyltransferase transposon mutants and the cpe2071 gene supplemented with plasmid loading (as described in Example 1) with and without proteinase K treatment are shown. Western blot against Clostridium perfringens of whole cell lysates prepared from Whole-cell lysates of four glycosyltransferase mutants (isolated from a previously described Clostridium perfringens HN13 transposon library (Liu et al. (2013)) were analyzed by Western blotting, and derived from Lysates of a cpe2071 gene-disrupted mutant (strain HLL8) did not contain the proteinase K-resistant antigen observed in the wild-type strain. Supplementation of this mutant with a plasmid-loaded copy of the cpe2071 gene resulted in restoration of the proteinase K-resistant antigen , confirmed that the loss of this antigen in the cpe2071 mutant was due to the disruption of the cpe2071 gene. Since cpe2071 encodes a polysaccharide and the ...
Embodiment 3
[0076] Formalin-fixed C. perfringens HN13 and JGS4143 cells were prepared for intramuscular (IM) injection into chickens as follows. Cells were grown overnight on PGY agar plates as described in Example 1. Cells from one plate of each were harvested and resuspended in 10 ml PBS, pelleted by centrifugation, resuspended in 10 ml PBS containing 1% (v / v) formalin, and incubated at 4°C for 2h. Wash cells 4 times in 2 ml PBS to remove formalin, then resuspend in PBS to OD 600nm is 1.0. The cell suspension was mixed 1:1 with Freund's complete adjuvant (FCA, initial injection) or Freund's incomplete adjuvant (FIA, booster injection). Broilers were given a primary injection (150 μl×2, IM, in the breast muscle) at 7 days of age, followed by a booster injection (150 μl×2, IM, in the breast muscle) at 21 days of age. Chickens were slaughtered and bled on day 35. The blood was allowed to clot overnight at room temperature and the next day the samples were centrifuged at 13 000 x g and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com