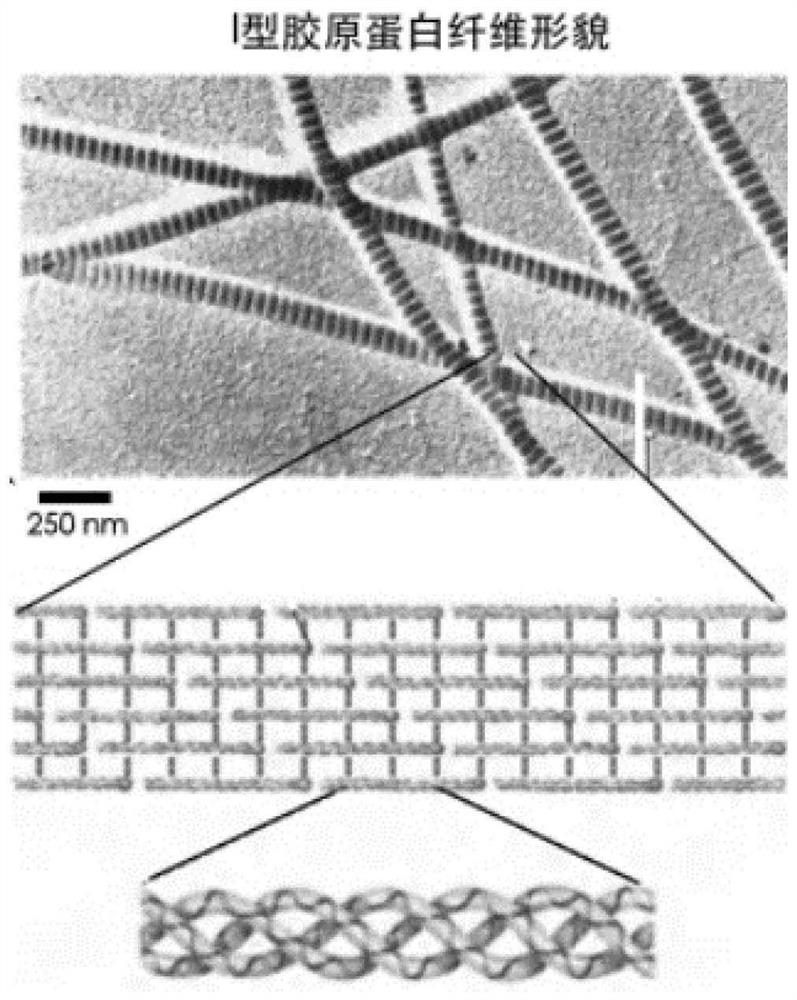
A method for regulating the period length of type I collagen fiber striations
A collagen fiber and collagen technology, which is applied in the field of genetic engineering, can solve the problems of high culture cost, long cycle, limited application and the like, and achieve the effect of simple preparation process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Sequence design and sample preparation
[0052] according to The structure shown is designed, and the specific steps are:
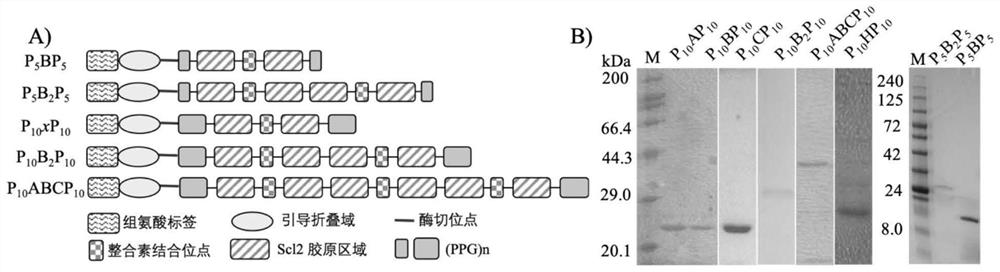
[0053] (1) With N and C terminals (GPP) 10 As a fixed sequence motif, a variable collagen region is inserted in the middle to obtain a three-segment chimeric sequence (abbreviated as P 10 CLP 10 ). In this example, the CL-domain adopts the amino acid sequence (abbreviation is H) as bacterial collagen, where the Scl2 collagen domain is divided into three equal-length domains A, B and C. In the following examples, the designed CL domains are A, B, C, BB (two repeated B regions) and ABC (equivalent to the complete Scl2 collagen region).
[0054] (2) A globular domain derived from Scl2 (shown in SEQ ID NO.1) is inserted at the N-terminus of the sequence to guide the correct folding of the collagen triple helix, and a protease cleavage site is inserted between the globular domain and the fixed sequence unit of the collagen region LVP...
Embodiment 2
[0060] Embodiment 2 sequence design and sample preparation
[0061] The specific implementation method is the same as in Example 1, the difference is that the amino acid length of the CL domain is increased, and the designed CL domains are BB (repeated 2 B regions) and ABC (equivalent to the complete Scl2 collagen region), and the corresponding complete amino acids The sequence and nucleotide sequence are:
[0062] V-P 10 B 2 P 10 The amino acid sequence is shown in SEQ ID NO.10, encoding V-P 10 B 2 P 10 The gene sequence of is shown in SEQID NO.16.
[0063] V-P 10 ABCP 10 The amino acid sequence is shown in SEQ ID NO.11, encoding V-P 10 ABCP 10 The gene sequence of is shown in SEQID NO.17.
[0064] The proteins prepared in Examples 1-2 were identified. figure 2 (B) shows that a single band can be detected by SDS-PAGE for the digested protein. Since collagen is a rod-shaped protein, the protein marker used is a spherical molecule, and the molecular weight shown by...
Embodiment 3 2
[0065] Example 3 Secondary Structure Determination
[0066] The collagen prepared in Examples 1-2 was prepared at a concentration of 1 mg / mL. Then stand at 4°C for more than 24h, use a 1mm cuvette, scan the full wavelength of circular dichroism at 4°C, the wavelength is from 190nm to 260nm, the wavelength interval is 1nm, and stay at each wavelength for 5s. The thermal change experiment was measured at 220nm, the temperature was from 4°C to 80°C, each temperature was equilibrated for 8s, and the temperature increment rate was 1°C / 6min. The typical CD spectrum of collagen triple helix structure shows a positive absorption peak at 220nm.
[0067] Such as Figure 4 As shown, under full-wavelength scanning, the proteins designed in Examples 1 and 2 have characteristic absorption peaks around 220nm; the results of thermal variation experiments show that, with the increase of temperature, the characteristic absorption values at 220nm are all abrupt at around 50°C. Changes, manife...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com