Organic electrolyte for producing hydrogen by electrolyzing hydrogen sulfide at low temperature, and cyclic reaction device and process
An organic electrolyte and electrolysis reactor technology, which is applied in the electrolysis process, electrolysis components, and bulk chemical production, etc., and can solve the problem of difficult separation of sulfur.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
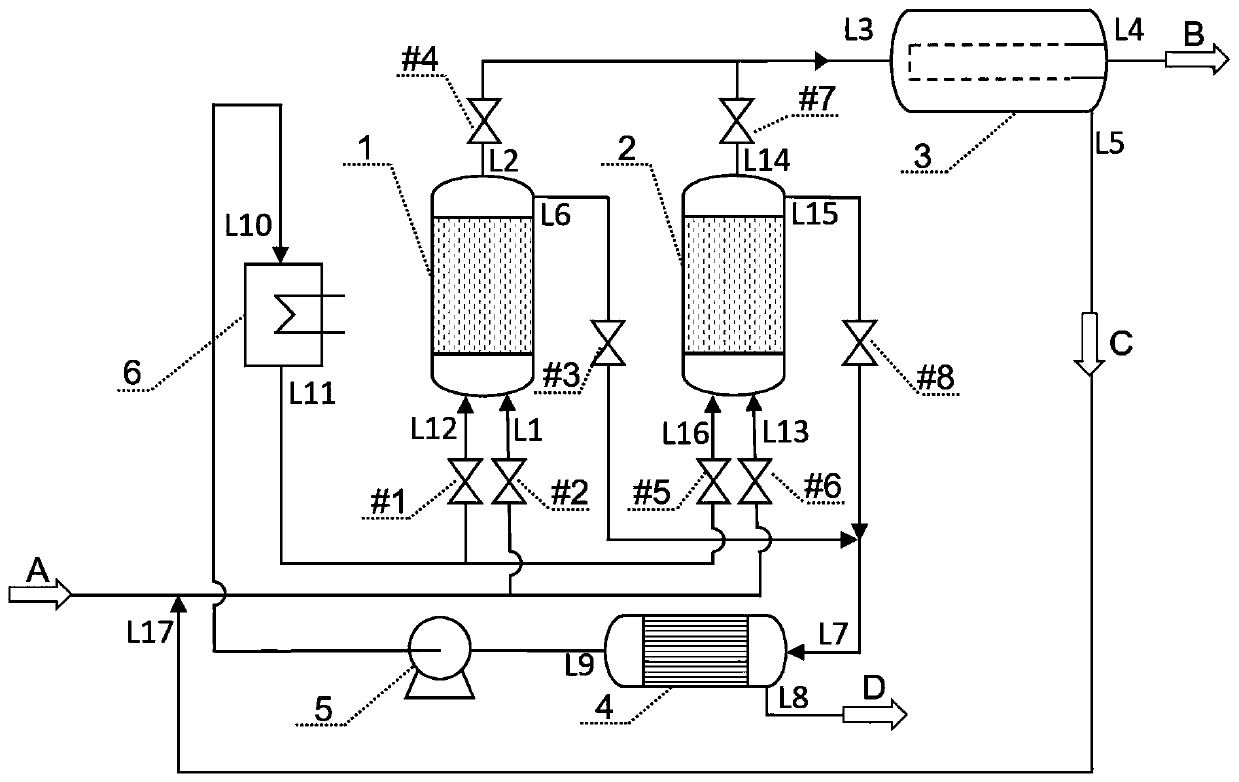
Image
Examples
Embodiment 1
[0072] (1) Configure 0.5mol / L[C 3 OHmin]BF 4 Tetraethylene glycol dimethyl ether solution is placed in five electrolytic cells, monoethanolamine MEA is added to make its mass fraction reach 1.5%, and the solution is mixed evenly;
[0073] (2) Infuse high-purity argon for 30 minutes to remove possible oxygen in the solution, while heating to 50°C;
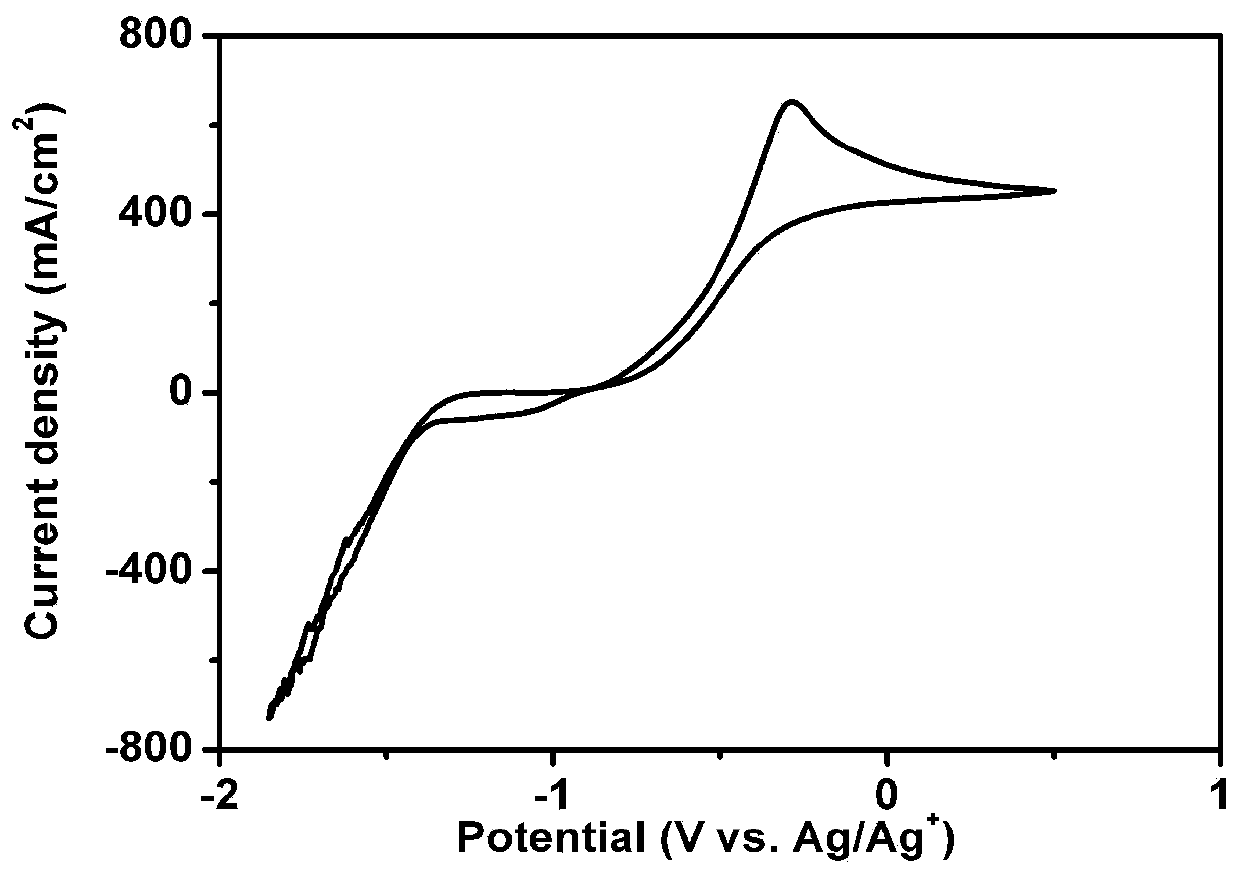
[0074] (3) Introduce hydrogen sulfide gas (which can be produced by the reaction of ferrous sulfide and dilute sulfuric acid or dilute phosphoric acid), and use a three-electrode system (working electrode, counter electrode, Ag / Ag + Reference electrode) carries out cyclic voltammetry test to the hydrogen sulfide in the solution, obtains the CV curve of the electrochemical behavior of the hydrogen sulfide in the solution, as figure 2 shown.
[0075] (4) Carry out constant potential electrolysis at 0V with respect to reference electrode, the current density time curve that electrolysis obtains, detect the hydrogen that generates w...
Embodiment 2
[0080] The difference from Example 1 is:
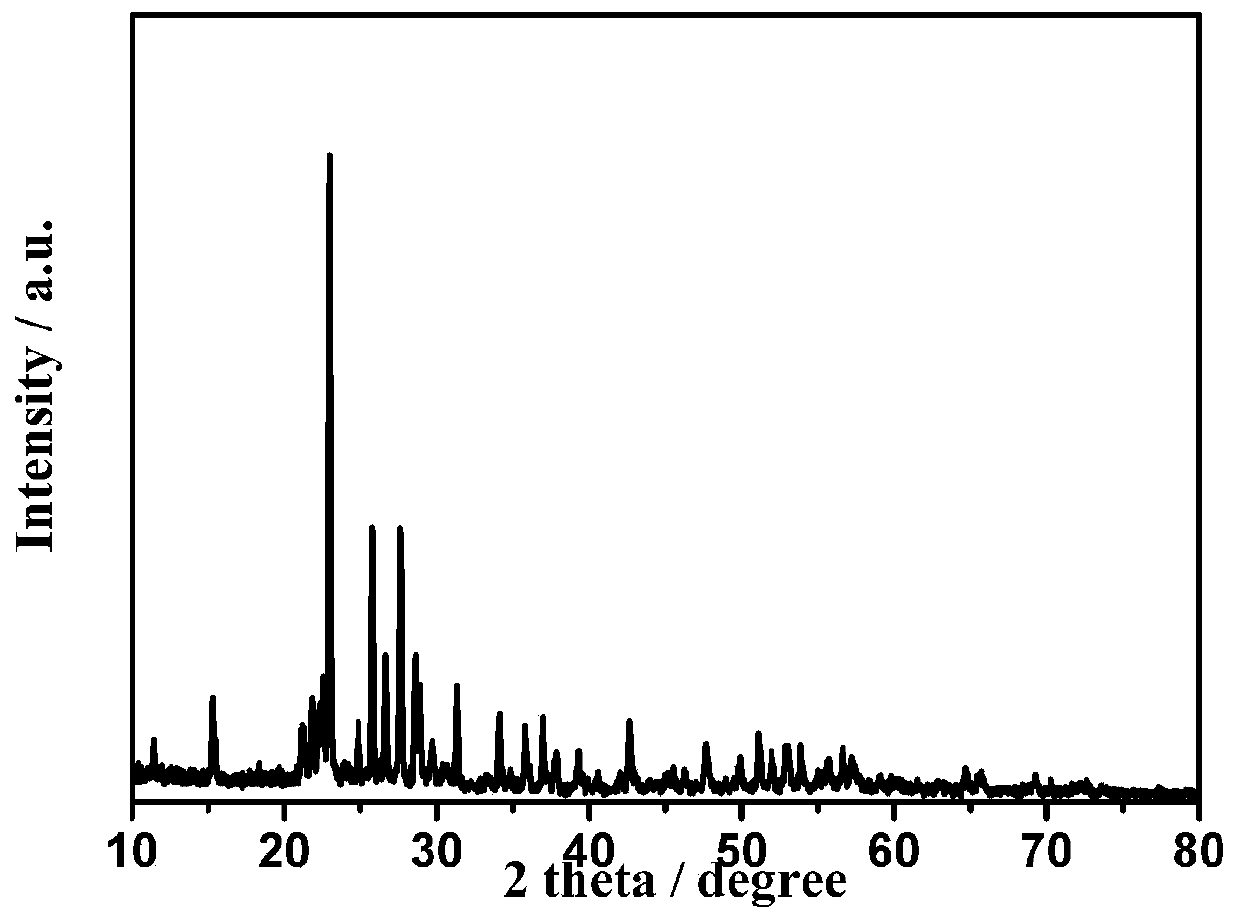
[0081] Configure 0.2mol / L[C 3 OHmin]BF 4 solution of tetraethylene glycol dimethyl ether. The obtained CV curve of the electrochemical behavior of hydrogen sulfide in solution, such as Figure 7 shown. The yellow solid XRD pattern after electrolysis is as follows Figure 8 shown.
Embodiment 3
[0083] The difference from Example 1 is that
[0084] Configuration 0.1mol / L[C 3 OHmin]BF 4 Add monoethanolamine MEA to make the mass fraction reach 1%, pass 30min high-purity Ar into the five-port electrolytic cell, and heat to 80°C, obtain hydrogen sulfide in the According to the CV curve in the system, the electrolysis voltage is determined to be -0.1V. Hydrogen sulfide is introduced into the system for electrolysis and it is found that there are bubbles in the cathode, that is, hydrogen gas is generated. The electrolysis reaction is carried out for 7 hours. After cooling the electrolyte, it is found that yellow solids are precipitated in the system. After centrifugal filtration , the solid and the supernatant were separated, the supernatant was further electrolyzed, the obtained solid was washed and dried, and the obtained solid was detected as elemental sulfur by XRD.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com