Preparation method of tetracycline carbon dot fluorescent molecular imprinting material
A carbon dot fluorescence and molecular imprinting technology is applied in the field of preparation of carbon dot molecularly imprinted fluorescent composite materials, which can solve the problems of complex and expensive instruments and equipment, and achieve the effects of easy control of reaction conditions, simple experimental operation and good stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
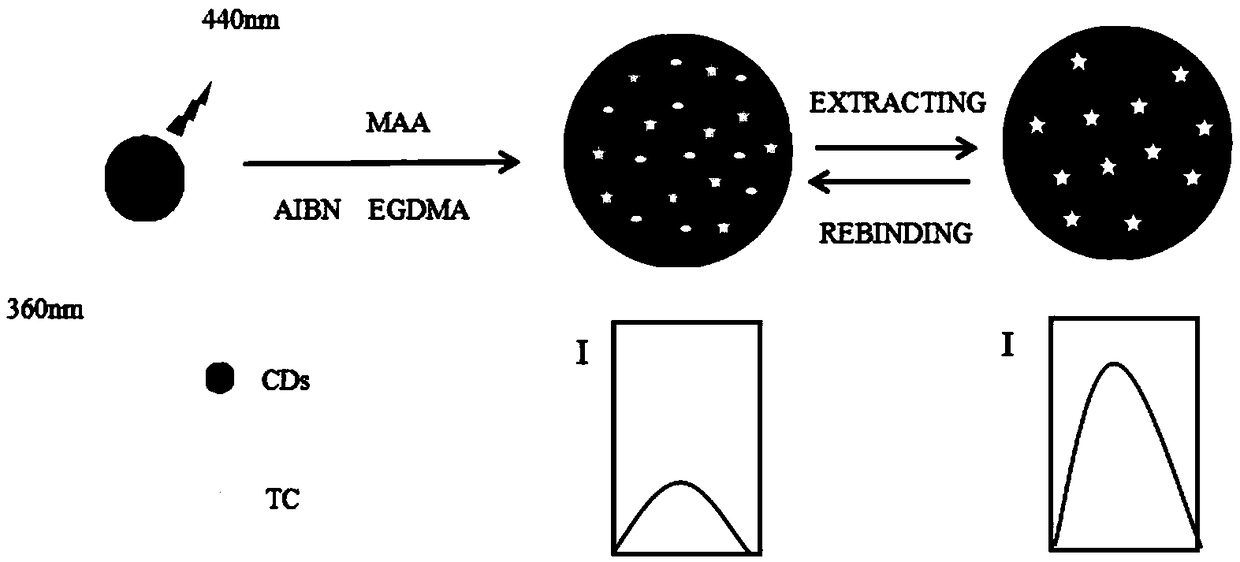
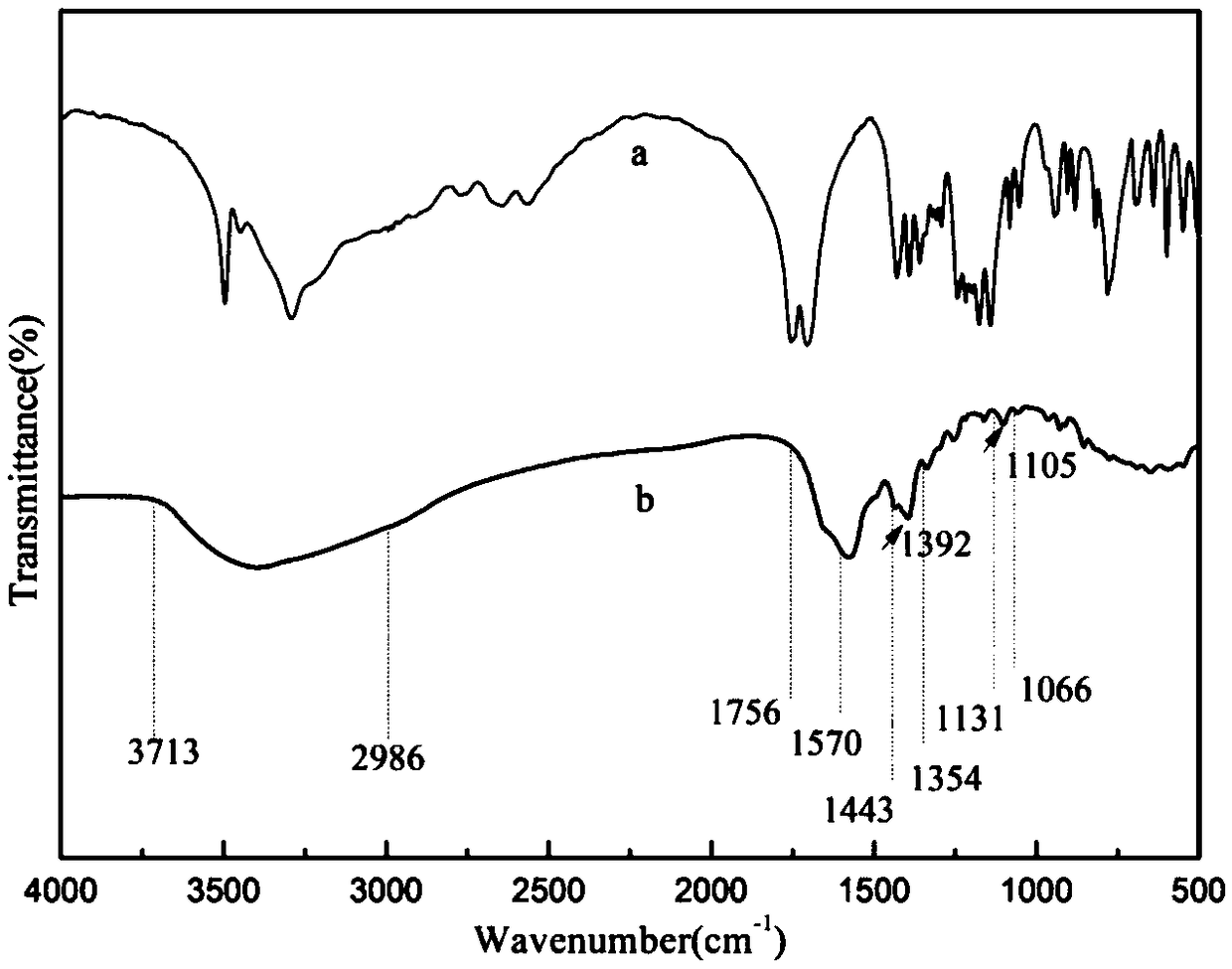
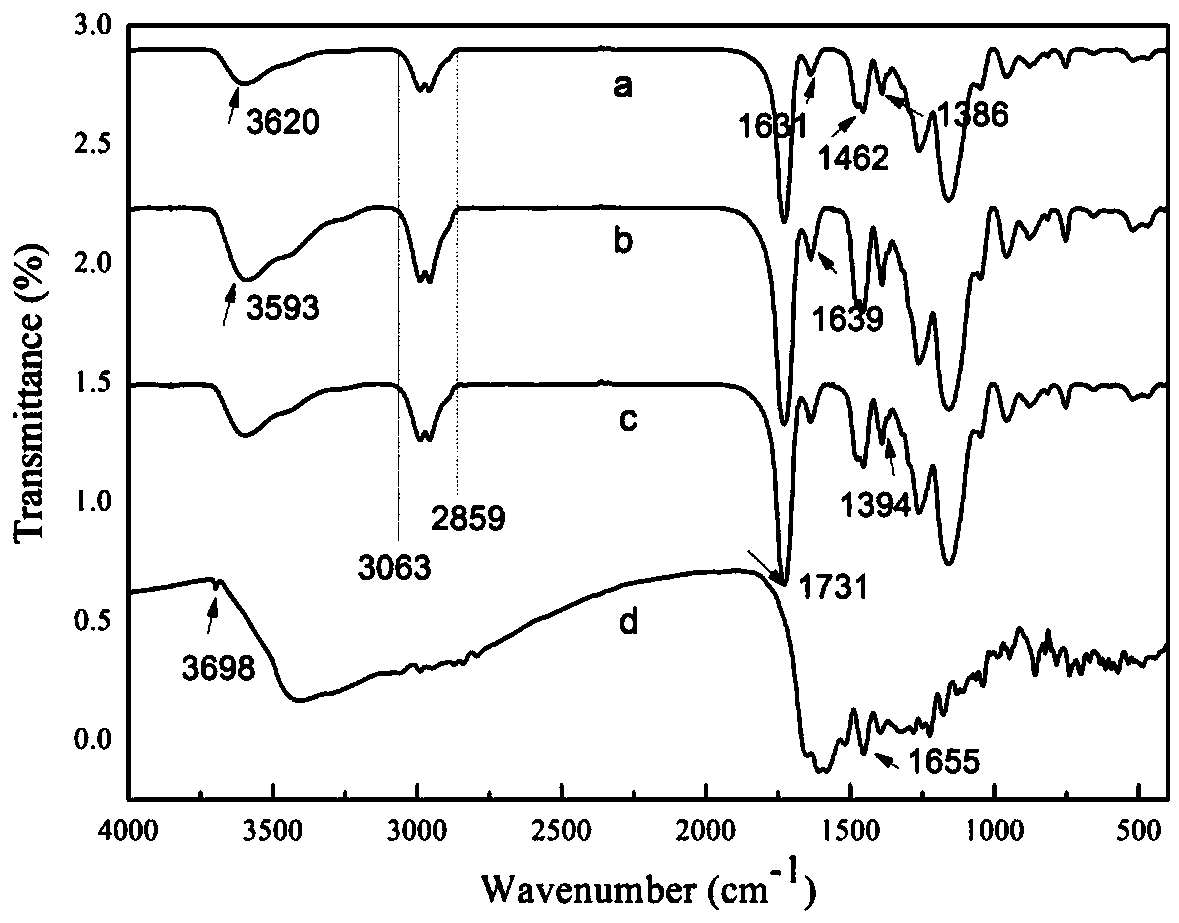
[0033] The invention provides a method for preparing a tetracycline carbon dot fluorescent molecularly imprinted composite material. The specific preparation route is as follows: figure 1 Shown:
[0034] (1) Dissolve 10g of citric acid (CA) in 40ml of deionized water, add 4.2g of magnesium hydroxide (Mg(OH) 2 ) and 5ml of ethylenediamine (EDA), stirred until the solution was colorless and clear, reacted in a reaction kettle at 200°C for 3 hours, and the resulting product was dialyzed for 48 hours in a dialysis bag and freeze-dried for 24 hours to obtain highly fluorescent carbon dots ( CDs), protected from light, sealed and stored in a refrigerator at 4°C.
[0035] (2) Add 3 mg of carbon dots (CDs) into a solution of 4.5 ml of methanol and 0.5 ml of deionized water, and ultrasonically treat it to dissolve and disperse evenly. Then add 44.443 mg tetracycline (TC), 0.8 mmol methacrylic acid (MAA), ultrasonic treatment, then add 7 mmol ethylene glycol dimethacrylate (EGDMA) and...
Embodiment 2
[0043] Research embodiment 1 obtains the adsorption performance of fluorescent composite material (such as Figure 6 shown). Put 1 mg of fluorescent molecular imprinted composite material in a 2 ml centrifuge tube, add 2 ml of different concentrations (0, 0.05, 5, 20, 40, 60, 80, 100, 140, 180 μg L -1 ) TC standard solution, shake at room temperature for 3.5h, and measure the fluorescence intensity value with a spectrofluorometer (λ=360nm). At the same time, the adsorption experiment of non-imprinted composite materials on TC was done in parallel. from Figure 6 It can be seen that with the increase of TC concentration, the fluorescence intensity weakens, and finally maintains a balance gradually. At the same concentration, the fluorescence quenching of CDs@MIP was greater than that of CDs@NIP.
Embodiment 3
[0045] Research Example 1 obtains the adsorption kinetics performance (such as Figure 7 shown). Weigh 1mg of fluorescent molecularly imprinted and non-imprinted composite materials into 2ml centrifuge tubes, add 2ml of 100μg L -1 TC standard solution, shake at room temperature for 0, 30, 60, 90, 120, 150, 180, 210, 240, 270, 300, 330 min respectively, and measure the fluorescence intensity with a fluorescence spectrophotometer. like Figure 7 As shown, the adsorption of the imprinted composite to the target almost reached equilibrium at 210 min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com