Acetophenone Derivatives Applied to Unsaturated Lipid Analysis Method
An analytical method and unsaturated technology, applied in the field of mass spectrometry, which can solve the problem that lipidomics is not widely used
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Analysis of C=C position on the fatty acid chain of phosphatidylcholine standard PC 16:0 / 18:1 (9Z) by sodium spray method
[0044] Preparation of analytical samples: 5 μM PC 16:0 / 18:1 (9Z) was dissolved in 1:1 acetonitrile:20 mM ammonium acetate aqueous solution, and the solution also contained 2 mM triFAP.
[0045] Mass Spectrometry:
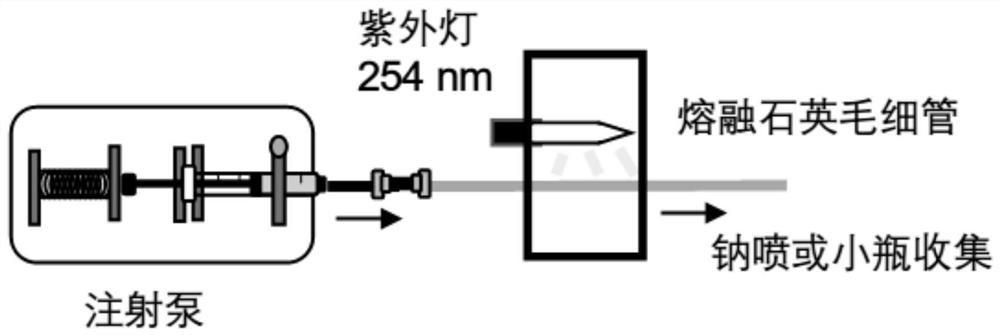
[0046] (1) The analytical sample PC 16:0 / 18:1 (9Z) was deoxygenated under nitrogen flow for about 5 minutes, and then loaded into the offline microfluidic reaction device. The reaction time is controlled at about 20s, and the reacted liquid is loaded into the sodium nozzle for mass spectrometry detection.
[0047] (2) Firstly, the unreacted solution is detected, and the stainless steel wire is inserted into the liquid of the analysis sample.
[0048] (3) In the positive ion PIS 184 mode, the lipid species was identified as phosphatidylcholine. Mass spectrometry parameters were set as follows: curtain gas 10psi; collision gas...
Embodiment 2
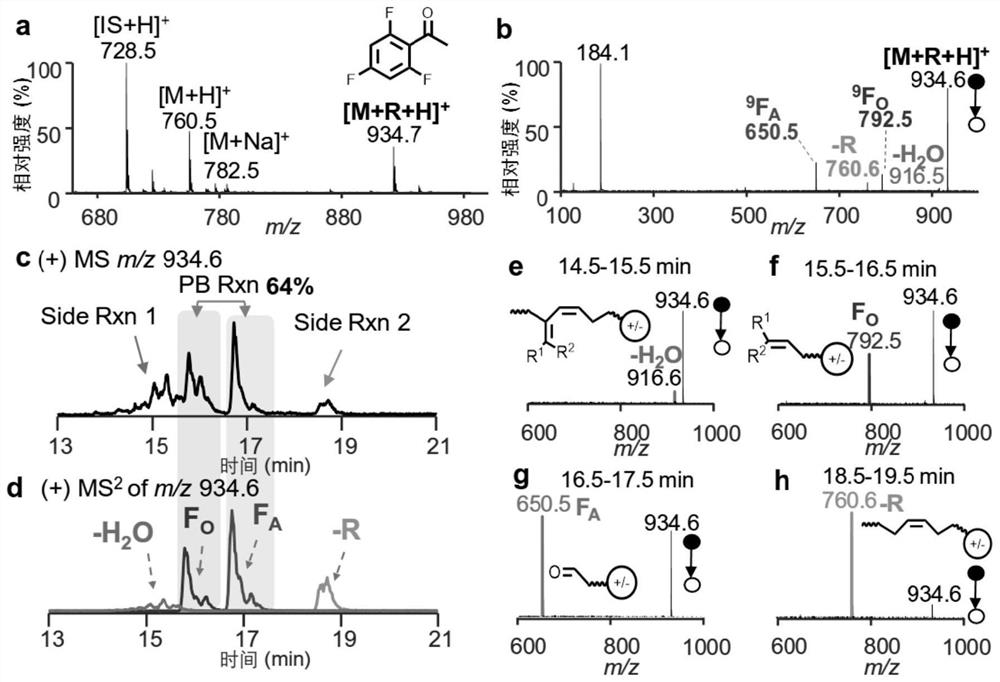
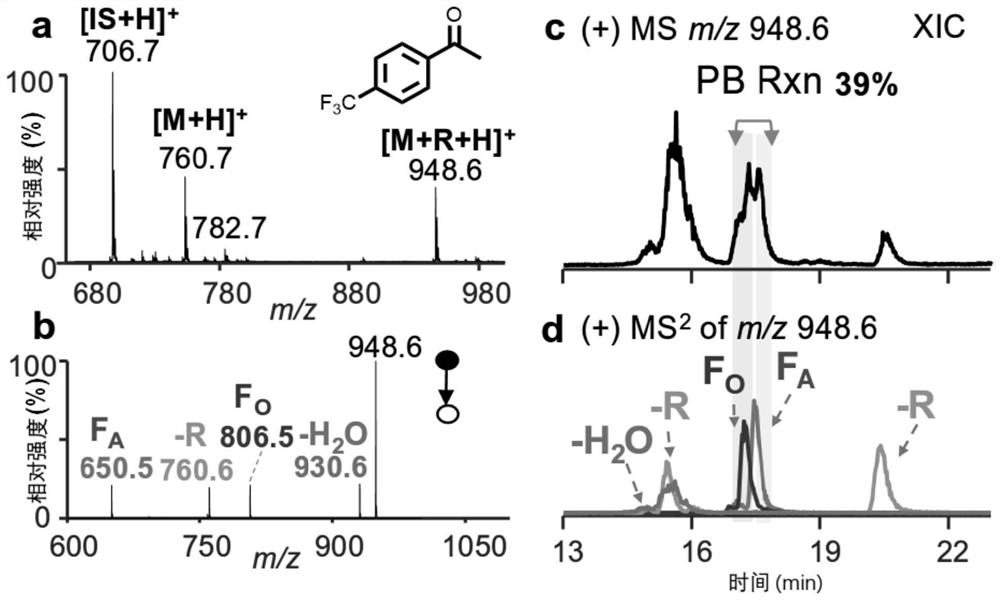
[0052]Mass spectrometry analysis of PC 16:0 / 18:1 was performed with reference to a method similar to that of Example 1, the difference being that FMAP was used for the acetophenone derivative. image 3 a is the primary mass spectrum after the reaction of PC 16:0 / 18:1 (5μM) and 2mM FMAP; image 3 b is the CID spectrum of the reaction product of PC 16:0 / 18:1 (collision energy is around 40eV); image 3 c is the chromatogram of the PC 16:0 / 18:1 reaction product separated by a reversed-phase chromatographic column, and the extracted ion is m / z 948.6 in the primary spectrum; image 3 d is the chromatogram of PC 16:0 / 18:1 reaction product separated by reversed-phase chromatographic column, the extracted ion is the fragment ion m / z 930.6(-H in the secondary spectrum 2 O), m / z 760.6 (-R), m / z 806.5 (F O ) and m / z 650.5 (F A ).
Embodiment 3
[0053] Example 3 Liquid Chromatography-Mass Spectrometry Analysis of the C=C Position of Lipid Molecules in Bovine Liver Lipid Extract
[0054] Preparation of analytical samples: 50 ppm bovine liver lipid extract dissolved in methanol
[0055] Mass Spectrometry:
[0056] (1) In the positive ion mode, neutral loss scans at 141Da, 185Da, 172Da and 260Da respectively obtained phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylglycerol (PG) and phosphatidylinositol (PI) Distribution information, the precursor ion scans m / z 184 to obtain the distribution information of PC (such as Figure 6 c). Mass spectrometry parameters were set as follows: curtain gas 30psi; collision gas high; voltage 4500V; temperature 400°C; spray gas 30psi; auxiliary heating gas 30psi; declustering voltage 80eV; collision energy 45eV. The liquid chromatography conditions are mobile phase A: 10 mM ammonium acetate aqueous solution, and mobile phase B: acetonitrile solution containing 0.2%...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| collision energy | aaaaa | aaaaa |
| collision energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com