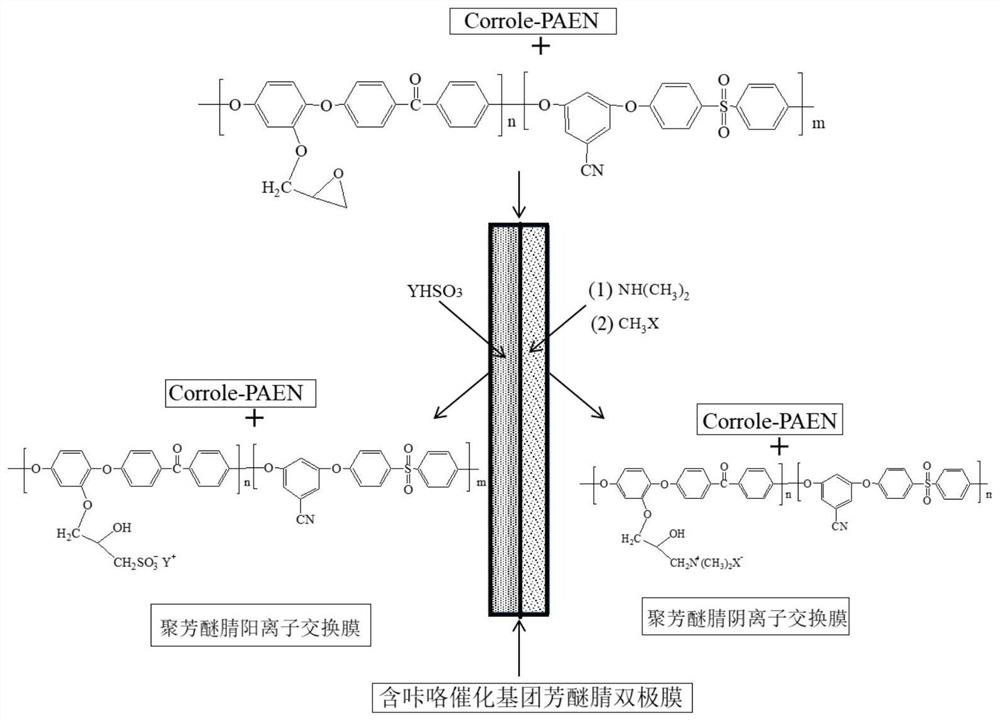
Preparation Method of Monolithic Polyarylether Nitrile Bipolar Membrane Containing Corrole Hydrodissociation Catalytic Group
A technology of polyarylether nitrile and bipolar membrane, which is applied in chemical instruments and methods, membrane technology, ion exchange, etc., can solve the problems of increased synthesis steps, limited use range, poor grafting uniformity, etc., and achieves low resistance and low cost. The effect of eliminating the film formation process and low transmembrane voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1) In a three-necked flask, add 2,4-dimethoxyhydroquinone 8.5570g (50mmol), resorcinol 3.3030g (30mmol), 2,4-difluoroterephthalonitrile 8.2055 g (50mmol), difluorodiphenyl sulfone 7.6275g (30mmol), anhydrous potassium carbonate 13.2664g (90mmol), toluene 320mL, DMAc 400mL, react at 140°C for 3h in a nitrogen atmosphere, then raise the temperature to 165°C for 10h, and immediately The reaction solution was poured into deionized water for precipitation under stirring, and filtered. The filtered precipitate was soaked in deionized water, soaked in running water for 24 hours, filtered, and dried in a vacuum oven at 80° C. for 10 hours to obtain a product containing methoxy polyarylether nitrile.
[0052]
[0053] (2) Weigh 5.0 g of polyarylether nitrile polymer containing methoxy in a 250 mL three-neck flask, install a constant pressure dropping funnel, and protect it with a nitrogen balloon after vacuuming. Add 120mL of pre-dried dichloromethane to dissolve the polyme...
Embodiment 2
[0065] (1) In a three-necked flask, add 8.6898g (70mmol) of o-methylhydroquinone, 1.3601g (10mmol) of 3-cyanoresorcinol, 15.2749g (70mmol) of difluorobenzophenone, and Fluorodiphenyl sulfone 2.5425g (10mmol), anhydrous potassium carbonate 13.2664g (90mmol), toluene 320mL, DMAc 400mL, react at 140°C for 3h in a nitrogen atmosphere, then raise the temperature to 160°C for 8h, and immediately pour the reaction solution under stirring into deionized water, and filtered. The filtered precipitate was soaked in deionized water, soaked in running water for 24 hours, filtered, and dried in a vacuum oven at 80° C. for 10 hours to obtain a product containing methoxy polyarylether nitrile.
[0066]
[0067](2) Weigh 5.0 g of polyarylether nitrile polymer containing methoxy in a 250 mL three-neck flask, install a constant pressure dropping funnel, and protect it with a nitrogen balloon after vacuuming. Add 120mL of pre-dried dichloromethane to dissolve the polymer, and add 3.6mL of bor...
Embodiment 3
[0079] (1) Preparation of polyarylether nitrile containing methoxy: in a three-necked flask, add 5.1369g (30mmol) of 2,5-dimethoxyhydroquinone, 1.1101g (10mmol) of resorcinol, two Fluorodiphenyl sulfone 7.6275g (30mmol), difluorobenzonitrile 1.3900g (10mmol), anhydrous potassium carbonate 6.9002g (50mmol), toluene 160mL, DMAc200mL, in N 2 React at 140°C for 3 hours in the atmosphere, then raise the temperature to 175°C for 9 hours, immediately pour the reaction solution into deionized water for precipitation under stirring, and filter. The filtered precipitate was soaked in deionized water, soaked in running water for 24 hours, filtered, and dried in a vacuum oven at 80° C. for 10 hours to obtain a product containing methoxy polyarylether nitrile.
[0080]
[0081] (2) Weigh 5.0 g of methoxy-containing polyarylether nitrile polymer into a 250 mL three-neck flask, install a constant-pressure dropping funnel, vacuumize, and protect with a nitrogen balloon. Add 120mL of pre-d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com