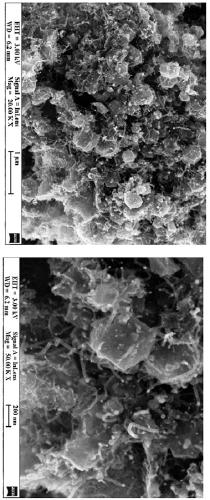
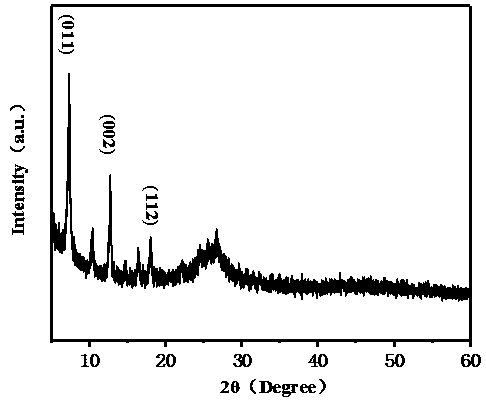
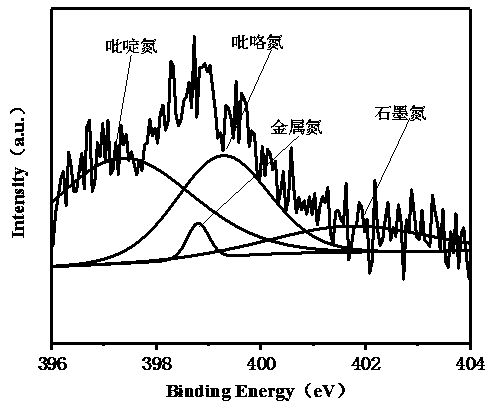
Cobalt-nickel bimetallic catalyst and preparation method thereof
A bimetallic catalyst, cobalt-nickel technology, applied in the field of cobalt-nickel bimetallic catalyst and its preparation, can solve problems such as poor stability, ethanol sensitivity, limited reserves, etc., and achieve the effects of preventing agglomeration, abundant active centers, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Weigh 1g of guanine, place it in a 30mL ceramic crucible, place it in a high-temperature tube furnace, heat it to 1000°C in a nitrogen atmosphere at a rate of 5°C / min, keep the temperature at a constant temperature for 2h, and wait for it to cool naturally to room temperature to obtain a porous carbon material. Weigh 150 mg of porous carbon, add it to a mixed solution of 40 mL of methanol and 40 mL of ethanol, and add 50 mg of F127 as a surfactant, and disperse it uniformly by ultrasonic treatment. Add 328mg of 2-methylimidazole under magnetic stirring. After stirring for 0.5 hours, add 292mg of cobalt nitrate hexahydrate, continue stirring for 0.5 hours, add 146mg of nickel nitrate hexahydrate, and let stand at room temperature for 24 hours. The product was collected by centrifugation and washed. Then, the product was dried in a vacuum oven at 60° C. overnight to obtain a black powdery solid. Finally, the product was put into a 30mL ceramic crucible, placed in a high...
Embodiment 2
[0028] Weigh 1g of guanine, place it in a 30mL ceramic crucible, place it in a high-temperature tube furnace, heat it to 1000°C in a nitrogen atmosphere at a rate of 5°C / min, keep the temperature at a constant temperature for 2h, and wait for it to cool naturally to room temperature to obtain a porous carbon material. Weigh 150 mg of porous carbon, add it to a mixed solution of 40 mL dimethyl sulfoxide and 40 mL ethanol, and add 50 mg of F127 as a surfactant, and disperse it uniformly by ultrasonic treatment. Add 328mg of 2-methylimidazole under magnetic stirring. After stirring for 0.5 hours, add 292mg of cobalt nitrate hexahydrate, continue stirring for 0.5 hours, add 146mg of nickel nitrate hexahydrate, and let stand at room temperature for 24 hours. The product was collected by centrifugation and washed. Then, the product was dried in a vacuum oven at 60° C. overnight to obtain a black powdery solid. Finally, the product was put into a 30mL ceramic crucible, placed in a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com