Process method for preparing glycolide from glycolate
A technology of glycolic acid ester and process method, which is applied in the direction of organic chemistry, can solve the problems of difficult separation and purification of glycolide, thermal deterioration of depolymerization solvent, and coking in the reactor, so as to shorten the reaction time and avoid solvent pollution , the effect of improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
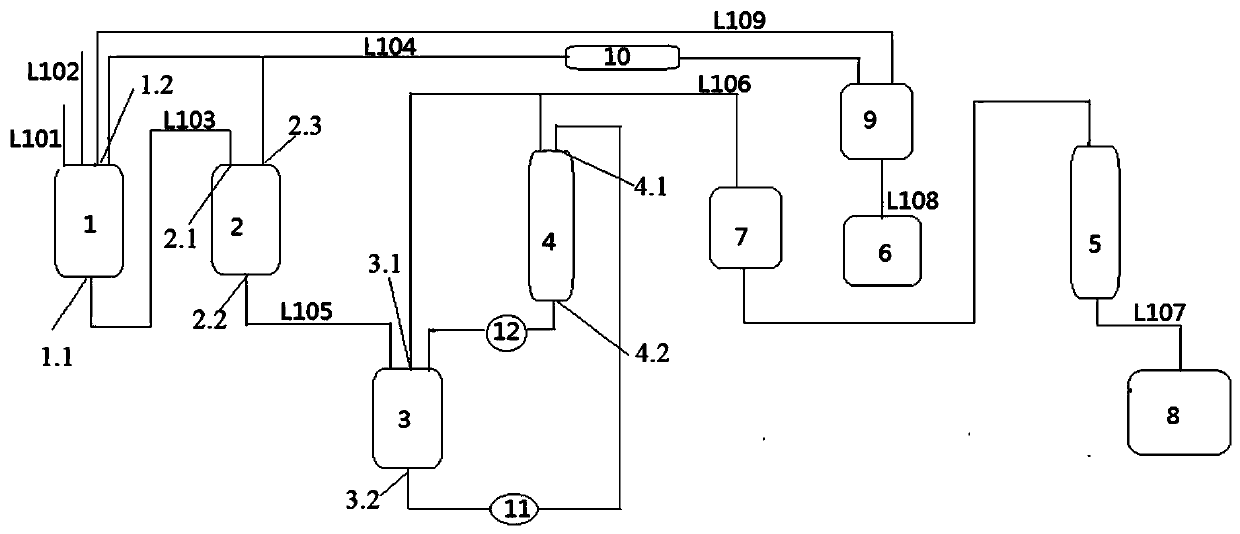
[0034] Add 600kg of methyl glycolate and 2.5kg of tin dichloride into the prepolymerization kettle 1, and control the reaction temperature at 140°C. During this process, methanol will be distilled out. After 1.5 hours of reaction, the reaction materials in the prepolymerization kettle 1 will be transferred to Continue the reaction in the final polymerization kettle 2, the vacuum state in the final polymerization kettle 2, the pressure 10KPa, the reaction temperature in the final polymerization kettle 2 is 140°C, the liquid evaporated from the prepolymerization kettle 1 and the final polymerization kettle 2 is collected by the distillation kettle 9, and the distillation kettle Separation and purification are carried out in 9, the low boiling matter methanol is condensed and collected by the recovery tank 6, and the high boiling matter is refluxed into the prepolymerization kettle 1 for continued use. The oligomers formed after the reaction of the materials in the final polymeriz...
Embodiment 2
[0036] Add 600kg of methyl glycolate and 3kg of stannous octoate into the prepolymerization tank 1, and control the reaction temperature at 140°C. During this process, methanol will be evaporated. After 1.5 hours of reaction, the reaction materials in the prepolymerization tank 1 will be transferred to the final polymerization tank. The reaction continues in kettle 2, the vacuum state in the final polymerization kettle 2, the pressure is 8KPa, the reaction temperature in the final polymerization kettle 2 is 140°C, the liquid evaporated from the prepolymerization kettle 1 and the final polymerization kettle 2 is collected by the distillation kettle 9, and in the distillation kettle 9 Separation and purification are carried out, and the low boilers are collected by the recovery tank 6 after the methanol is condensed, and the high boilers are refluxed into the prepolymerization kettle 1 for further use. The oligomers formed after the reaction of the materials in the final polymeri...
Embodiment 3
[0038] Add 600kg of methyl glycolate and 3.6kg of antimony trioxide into the prepolymerization kettle 1, and control the reaction temperature at 130°C. During this process, methanol will be distilled out. After 1 hour of reaction, the reaction materials in the prepolymerization kettle 1 will be transferred to the final The reaction continues in the polymerization kettle 2, the vacuum state in the final polymerization kettle 2, the pressure is 5KPa, the reaction temperature in the final polymerization kettle 2 is 150°C, the liquid evaporated from the prepolymerization kettle 1 and the final polymerization kettle 2 is collected by the distillation kettle 9, and the distillation kettle 9 Separation and purification are carried out in the middle, the low boiling matter methanol is condensed and collected by the recovery tank 6, and the high boiling matter is refluxed into the prepolymerization kettle 1 for further use. The oligomers formed after the reaction of the materials in the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com