Human thyroid stimulating hormone receptor fusion protein as well as preparation method and application thereof
A technology of thyroid stimulating hormone and fusion protein, applied in the biological field, can solve problems such as low expression and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Using CHO cells to construct a system for transiently expressing fusion proteins
[0031] (1) Construction of a plasmid containing the target gene
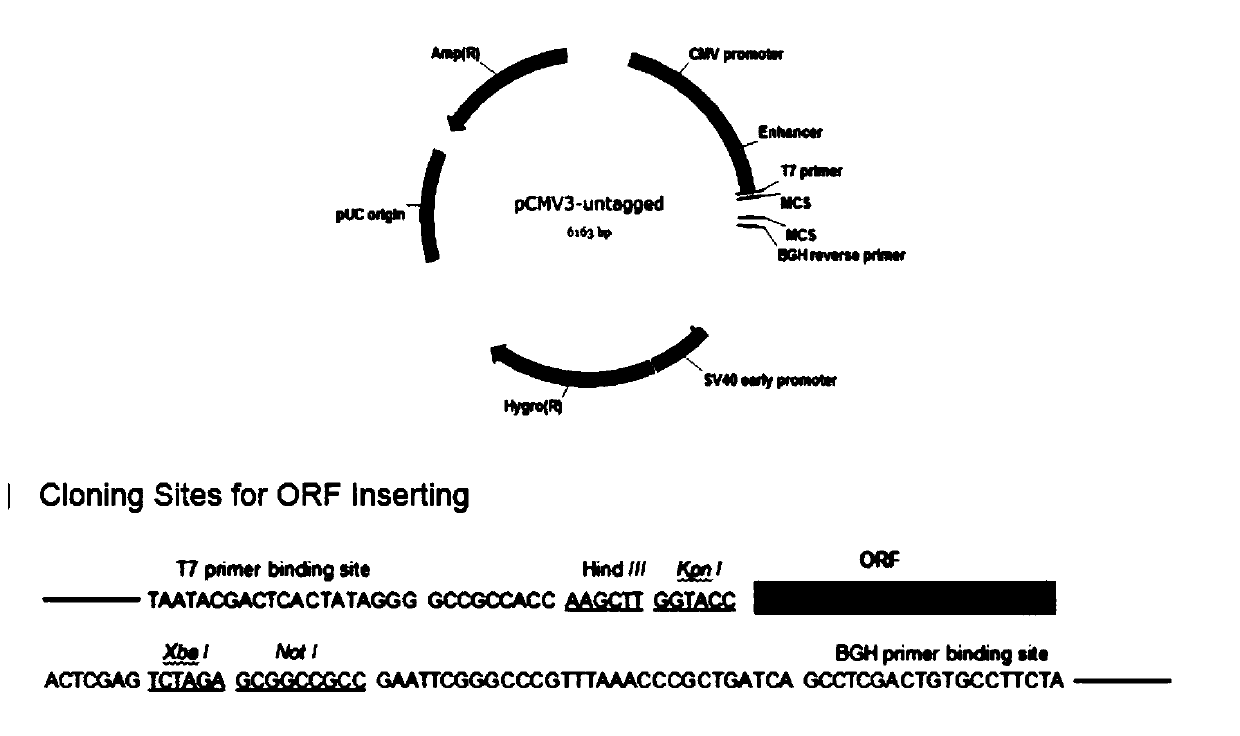
[0032] According to the experimental requirements, the PCMV3 plasmid was selected as the backbone, and the TSHR target gene fragment was inserted into the multiple cloning site. The PCR systems in Table 1 and Table 2 and the PCR program in Table 3 were used to amplify the gene fragments of HER2 and TSHR, respectively.
[0033] Table 1 PCR system
[0034]
[0035]
[0036] Table 2 PCR system
[0037]
[0038] Table 3 PCR program
[0039]
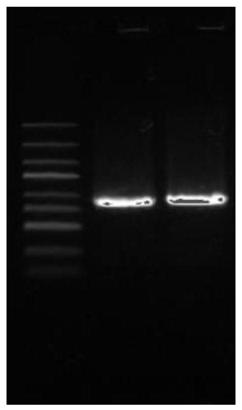
[0040] Glue recovery:
[0041] The DNA fragments were run on 1% DNA agar gel, and then recovered with a recovery kit, and then splice PCR was performed.
[0042] Splicing PCR:
[0043] The primer information is as follows. After PCR, the gel is recovered, and then digested.
[0044] Table 4 PCR system
[0045]
[0046]
Embodiment 2
[0137] Example 2 Constructing a system for stably expressing fusion proteins with CHO cells
[0138] (1) Construction of PMKO-TSHR-HER2 plasmid
[0139] The TSHR-HER2 fragment was inserted into the PMKO plasmid from the plasmid, and the method was as described in Experiment 1 above.
[0140] (2) Preparation of virus
[0141] The constructed plasmids and viral packaging plasmids VSVG and GAG were transfected into CHO cells with PEI. After 12 hours of transfection, the cell culture medium was removed, washed twice with PBS, and replaced with fresh cell culture medium. Collect the virus 36 hours after changing the medium, store it at 4°C within a week, and store it at -80°C for a long time.
[0142] (3) Virus infects target cells
[0143] Add 4 ml of virus to a 10 cm dish, add 2 ml of virus to a 6 cm dish, add 1 ml of virus to a 3.5 cm dish, and add polybrene to increase the efficiency of virus infection. After 5 hours of infection, add fresh cell culture medium, add 4 ml of...
Embodiment 3
[0148] Embodiment 3 purifies fusion protein
[0149] The purification method of the fusion protein utilizes the principle of his column metal chelation chromatography. The operation method is as follows:
[0150] Buffer:
[0151] Loading buffer: 0.02M phosphate, 0.5M NaCl 5-50mM imidazole pH7.4
[0152] Elution buffer: 0.02M phosphate, 0.5M sodium chloride, 0.5M imidazole pH7.4.
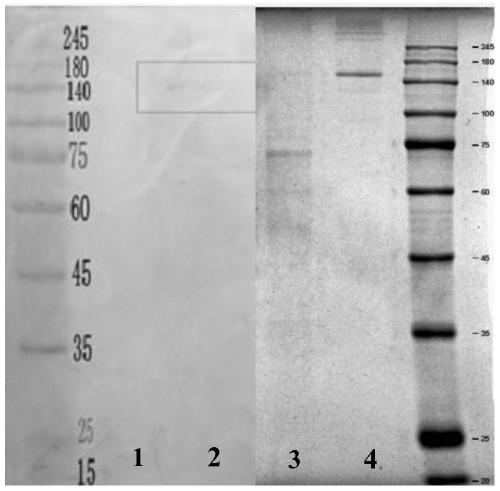
[0153] 1) Collect the cell supernatant, and then take a sample for SDS-PAGE electrophoresis analysis.
[0154] 2) Concentrate the supernatant in a hollow fiber column, and then take a sample for SDS-PAGE electrophoresis analysis.
[0155] 3) Chromatographic column preparation: use 0.1M nickel sulfate (NiSO 4 ) to fill the column with Ni 2+ , and then flush at least 5 column volumes with ultrapure water.
[0156] 4) Sample purification steps:
[0157] a. Equilibrate 5-10 column volumes with loading buffer.
[0158] b. Sample loading.
[0159] c. Wash 5-10 column volumes with loading buffer. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com