Transaminase mutant derived from aspergillus terreus and application of transaminase mutant
A transaminase and mutant technology, applied in the field of biopharmaceuticals, can solve the problems of easy racemization, affecting the optical purity of products, etc., and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
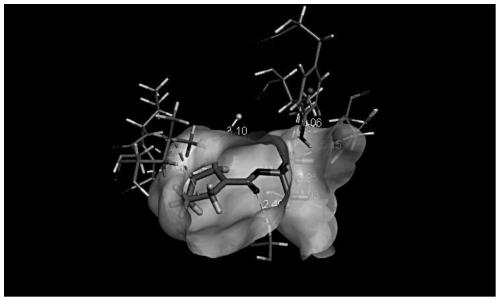
[0040] Example 1: Molecular docking evaluation of candidate transaminases and reaction substrate N-BOC-3-pyridone
[0041]Homologous protein modeling and evaluation are performed on the proteins that have not been analyzed for the tertiary structure in the present invention, and the three-dimensional transaminase model required by the present invention is obtained. The reaction substrate N-BOC-3-pyridone was molecularly docked with candidate transaminases. The RMSD threshold was set at 0.5 angstroms to ensure that the docking conformation was as diverse as possible, and the docking method with the highest scoring function was selected. 12 candidate transaminases of the present invention were comprehensively evaluated, and 6 candidate transaminases including Aspergillus terreus NIH2624 transaminase were selected for subsequent transaminase activity tests.
Embodiment 2
[0042] Example 2: Activity testing of transaminases with higher evaluation scores based on molecular docking results
[0043] The six candidate transaminase mRNA sequences were codon-optimized and related regulatory sequences were added (with BglII and XhoI endonuclease gene fragments on both sides of the fragments) for gene synthesis.
[0044] Recombinant plasmid transfection:
[0045] 1. Take Escherichia coli competent cells BL21 from -80°C and thaw in an ice box at room temperature.
[0046] 2. Add 1 ul plasmid to the competent cells and place in a 4°C refrigerator or ice box for 30 minutes.
[0047] 3. Heat shock in a water bath at 42°C for 120s, and immediately place it on an ice box for 2 minutes.
[0048] 4. Add 900 ul of recovery medium to each tube, shake at 150 rpm, and incubate at 37°C for 45 minutes.
[0049] 5. Centrifuge at low speed to take 50ul of the transformation solution from the bottom of the tube and spread it on a petri dish containing kan at the work...
Embodiment 3
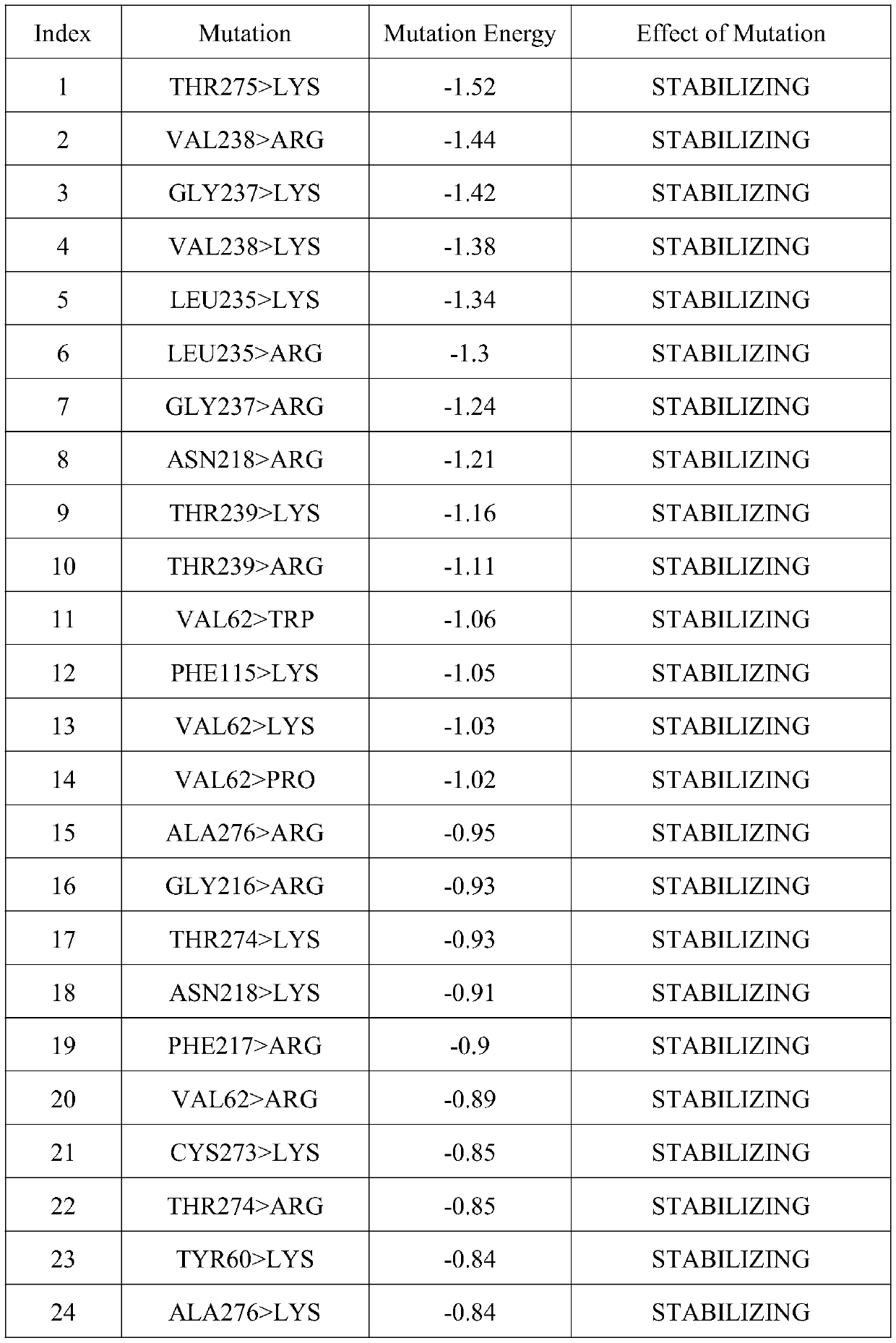
[0061] Example 3: Determining the amino acid mutation site by virtual mutation of a single amino acid around the active center of Aspergillus terreus NIH2624 transaminase
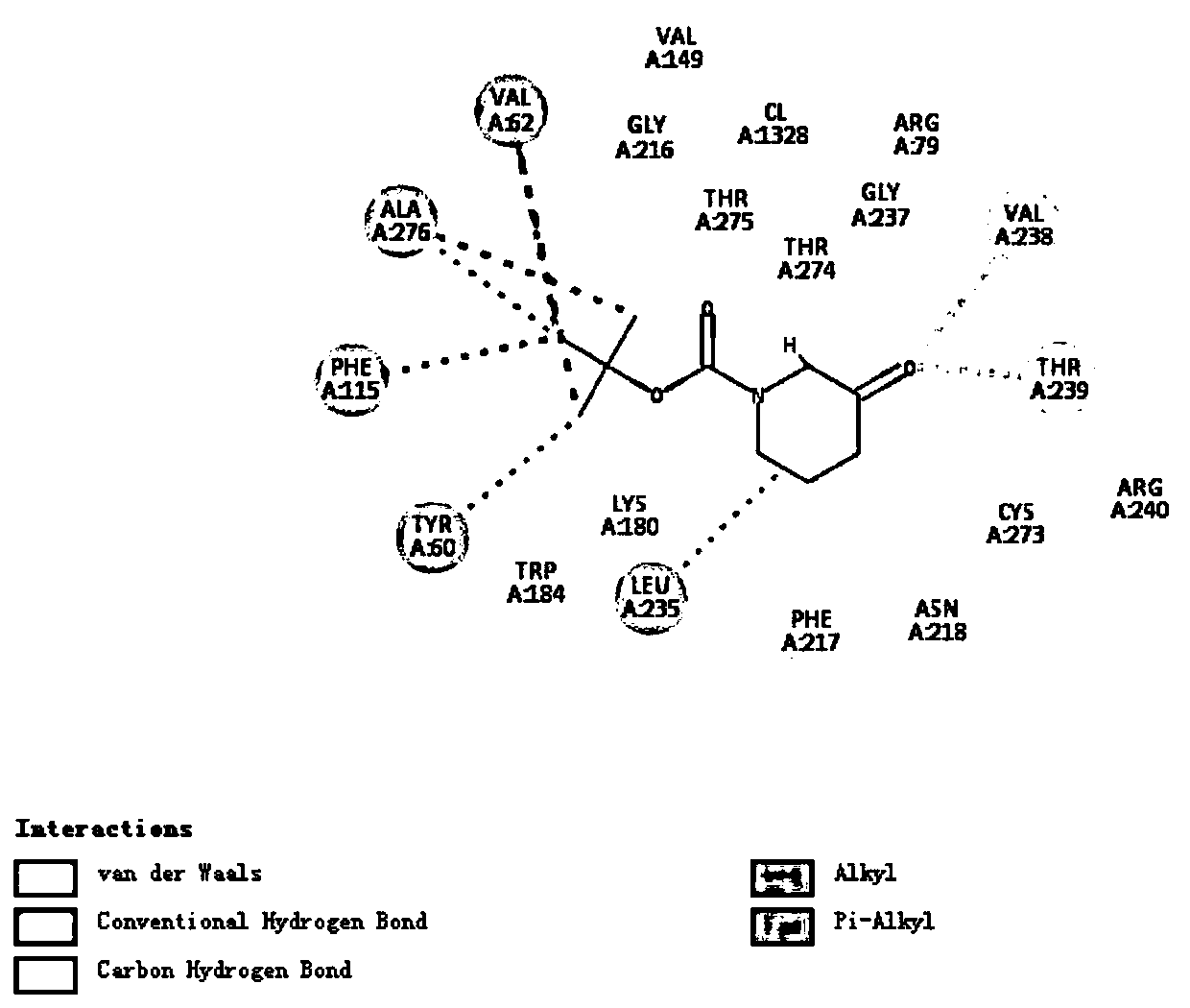
[0062] The present invention performs virtual mutation on Aspergillus terreus NIH2624 transaminase, uses molecular simulation software to rationally design Aspergillus terreus NIH2624 transaminase, and selects mutation sites, which can effectively save the time for mutation site screening and improve mutation efficiency.
[0063] The present invention uses the docking results in Example 1 to perform data analysis to determine the spatial distance and action relationship of each amino acid in the active region.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com