Catalyst for preparing cyclic carbonate compound by taking CO2 as raw material
A cyclic carbonate and catalyst technology, applied in organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, chemical/physical process and other directions, can solve problems such as poor circular economy and achieve high-efficiency reaction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
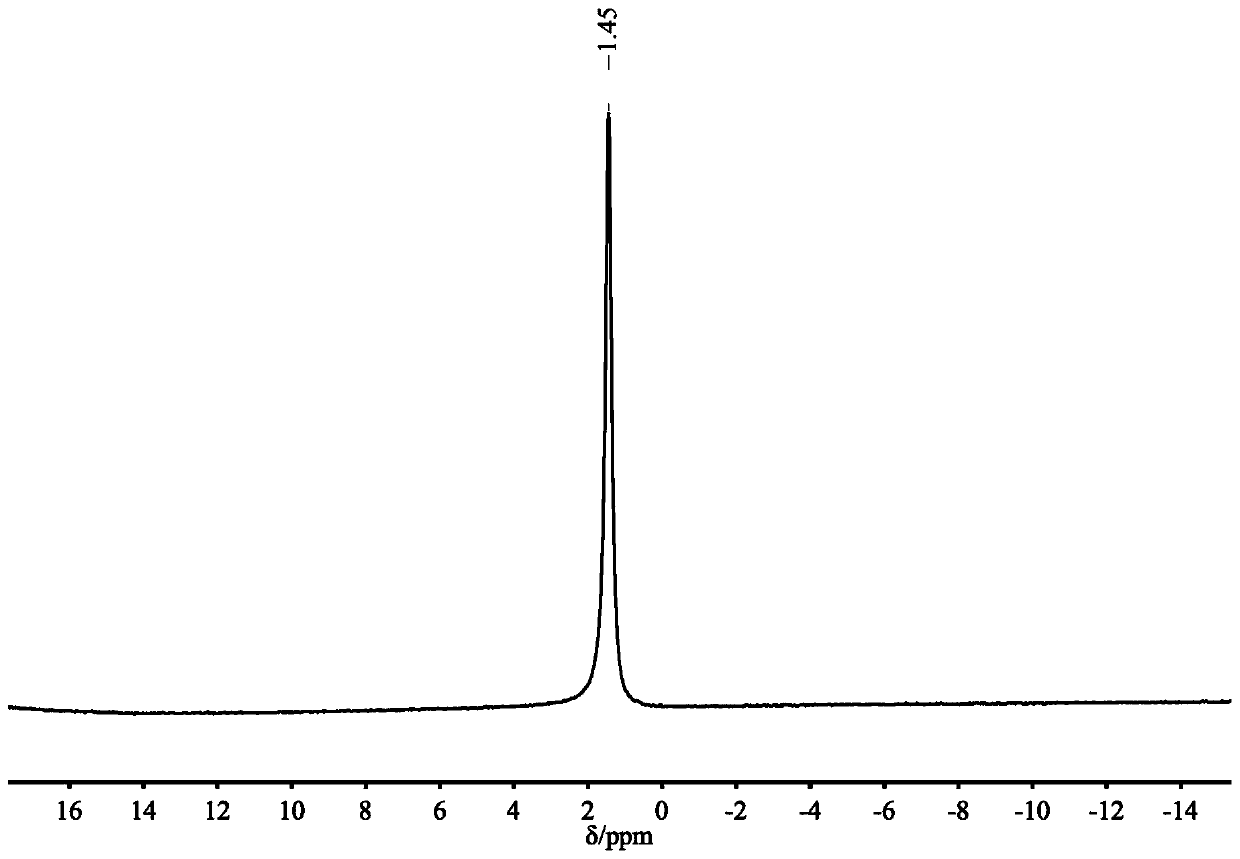
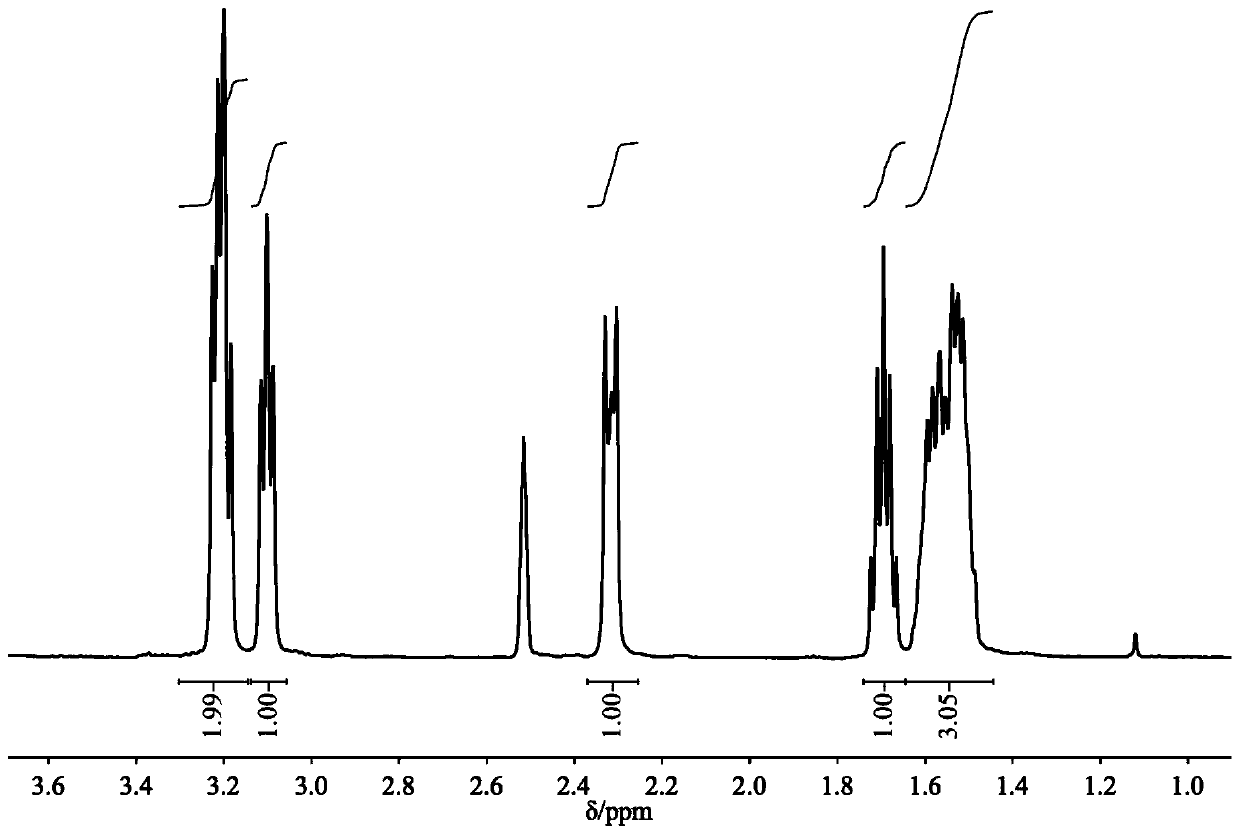
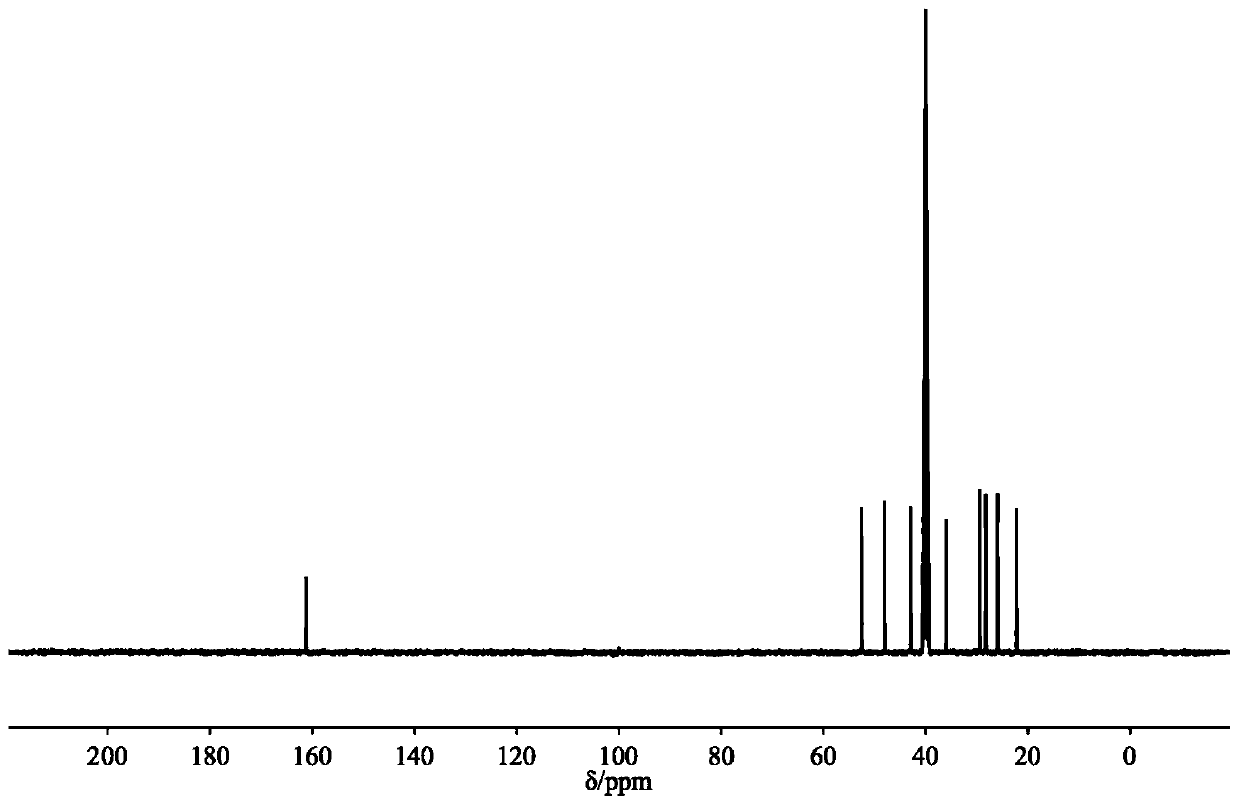
[0027] Example 1: Preparation of DBUHBO 2 , mix 1.5mmol DBU with an equivalent amount of HBO 2 Add them together into a 22mL stainless steel autoclave with polytetrafluoroethylene lining, seal the autoclave, vacuumize it, put it into an oil bath at a constant temperature, and stir at a constant speed to carry out the synthesis reaction. After the reaction, use a deuterated reagent as a solvent for nuclear magnetic resonance spectrum detection, and the boron spectrum is as follows: figure 1 As shown, a single peak appears at 1.45ppm, indicating that there is only one form of boron in this substance, namely BO 2 - is the only form; H NMR spectrum such as figure 2 As shown, similar to the H NMR spectrum of DBU, the C NMR spectrum is as image 3 As shown, the number of carbon atoms is also consistent with DBU, indicating that DBU as a cation donor does not change the carbon skeleton structure after the reaction; the infrared spectrum is as follows Figure 4 As shown, the N-H...
Embodiment 2
[0028] Embodiment 2: Preparation of chloromethyl dioxolanone, reaction flow diagram sees Image 6 . Add 2mmol epichlorohydrin and a specific amount of ionic liquid into a 22mL stainless steel autoclave with a polytetrafluoroethylene liner, seal the autoclave, and fill it with a certain pressure of CO after vacuuming. 2 Gas, put the reactor into a constant temperature oil bath, stir, and carry out the synthesis reaction. After the reaction, the yield of chloromethyldioxolanone can be calculated by using the deuterated reagent as the solvent and N,N-dimethylformamide (DMF) as the internal standard by proton nuclear magnetic resonance spectroscopy.
Embodiment 3
[0029] Embodiment 3: In order to investigate the reactivity of different catalysts to catalyze the generation of chloromethyl dioxolanone, the following experiments were carried out, and the reaction conditions were: epichlorohydrin (2mmol), different catalysts (1.5mmol), CO 2 The pressure is 0.1MPa, the reaction temperature is 30°C, and the reaction time is 4h. The results are shown in Table 1. DBUHBO 2 best catalytic activity.
[0030] The impact of different catalysts on the reaction yield in table 1
[0031]
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com