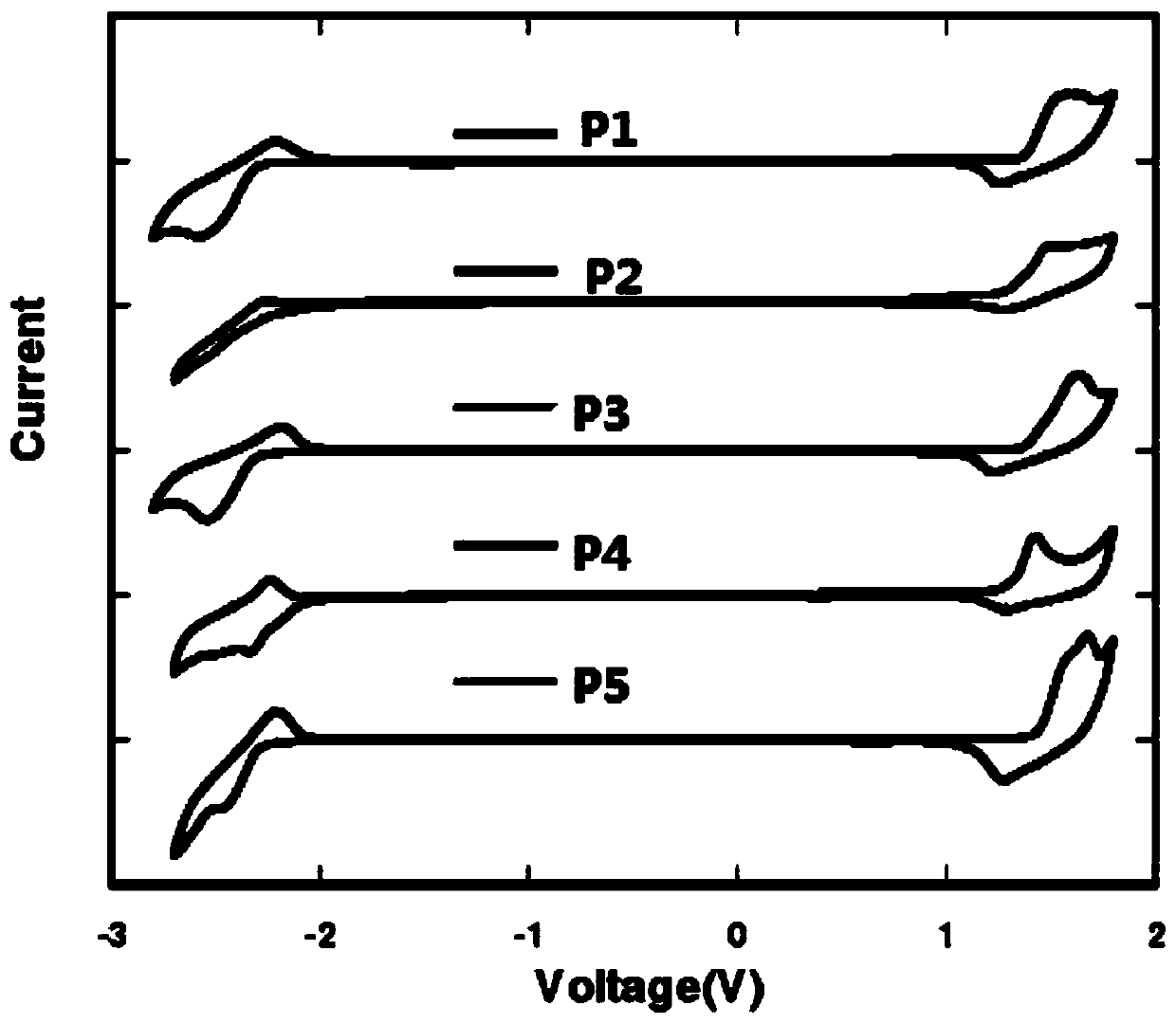
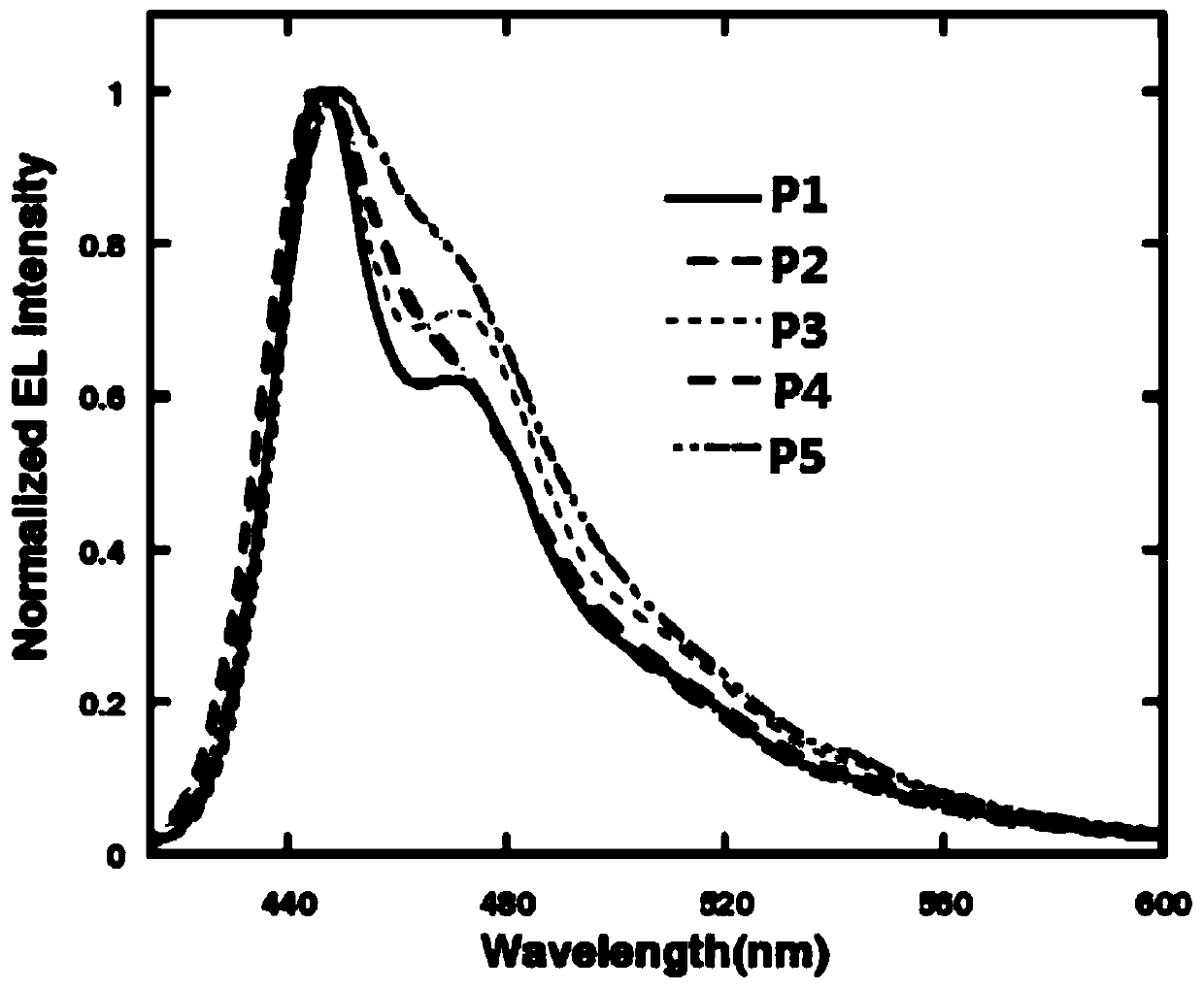
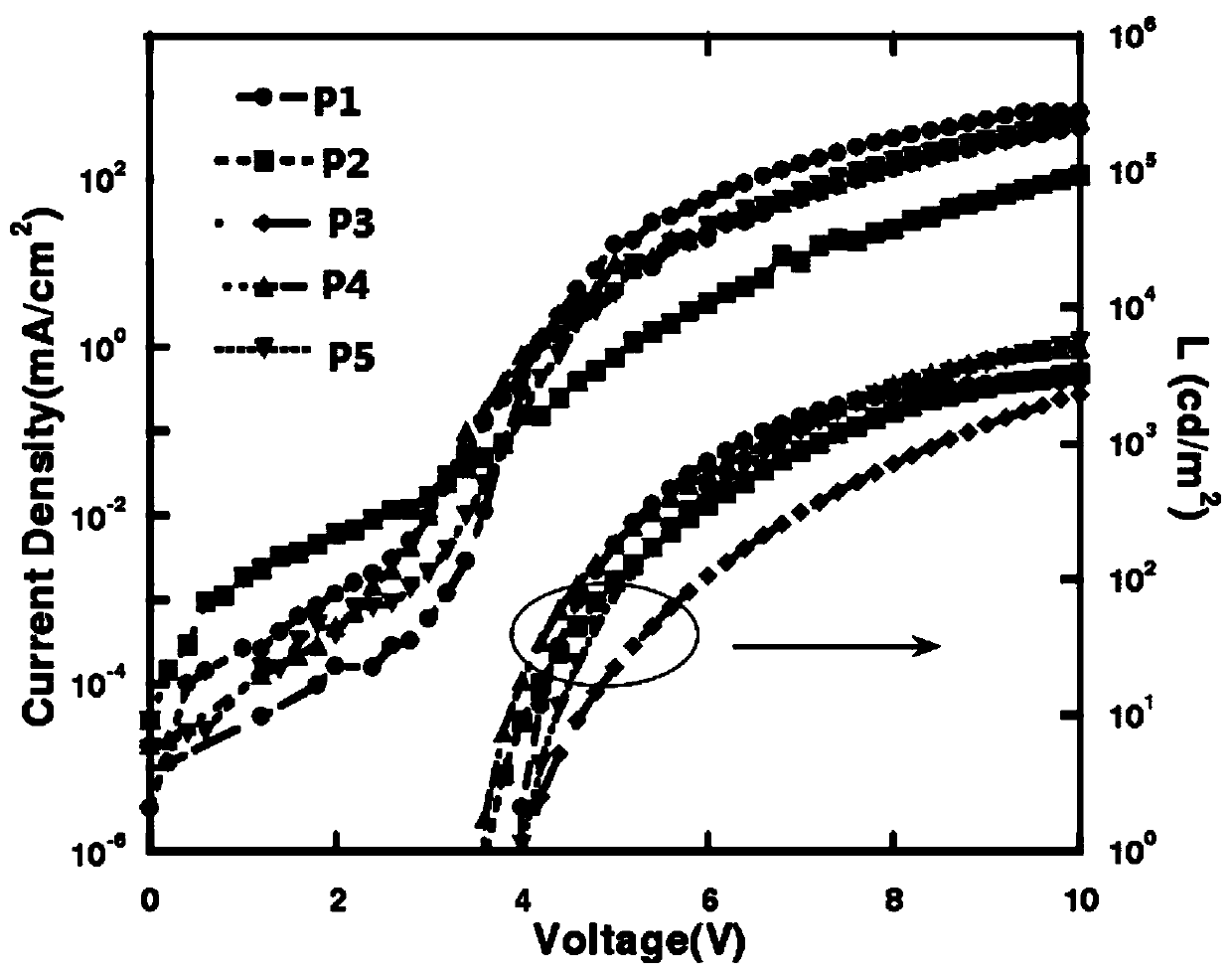
Thiazole-based organic electroluminescent material and preparation method thereof
An electroluminescent material and organic technology, applied in the direction of luminescent materials, chemical instruments and methods, circuits, etc., can solve the problems of difficult to balance hole and electron transport efficiency, poor electroluminescence efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Under nitrogen protection, 9,9-bis(4-tetrazolylphenyl)fluorene (1.5g, 3.3mmol) and 15mL pyridine were added to a 50ml two-necked flask, and 4-tert-butylbenzene was added dropwise to the mixed solution. Formyl chloride (2 mL, 10 mmol). The reaction mixture was heated at 130 °C for 4 hours. After the reaction was completed, the reaction solution was poured into a mixture of water and MeOH (150 mL, 1:3), dried, filtered, and the solvent was removed by a rotary evaporator. The crude product was subjected to silica gel column chromatography (the eluent was petroleum ether: ethyl acetate = 4:1), and in toluene / CHCl 3 Recrystallization in (5:1) yielded 5,5'-(((9H-fluorene-9,9-diyl)bis(4,1-phenylene))bis(2-(4-(tert Butyl) phenyl)-1,3,4-oxadiazole) (2.3g, yield 90.3%), its reaction equation is as follows:
[0029]
Embodiment 2
[0031] Under a nitrogen atmosphere, add 5,5'-(((9H-fluorene-9,9-diyl)bis(4,1-phenylene))bis(2-(4 -(tert-butyl)phenyl)-1,3,4-oxadiazole) (0.5g, 0.70mmol) and CHCl 3 (50mL), stir well and add trifluoroacetic acid (0.5g, 1.16mmol) and bromine (0.24gg, 1.50mmol) to the mixed solution. The reaction mixture was stirred at 50 °C for 48 h, then washed with 100 ml NaHCO 3 Wash with aqueous solution until the red color of bromine disappears. The organic layer was washed with Na 2 SO 4 Dry and remove solvent in vacuo. The residue passes through CH 2 Cl 2 / ethyl acetate (20:1) eluent was subjected to silica gel column chromatography to obtain 5,5'-(((2,7-dibromo9H-fluorene-9,9-diyl)bis(4, 1-phenylene))bis(2-(4-(tert-butyl)phenyl)-1,3,4-oxadiazole) (0.4g, yield 65.0%), the reaction equation is as follows:
[0032]
Embodiment 3
[0034] 5,5'-(((2,7-dibromo9H-fluorene-9,9-diyl)bis(4,1-phenylene))bis(2-(4-(tert-butyl)benzene base)-1,3,4-oxadiazole) (11.36g, 15mmol), 2-isopropyl-4,4',5,5'-tetramethyl-1,3,2-dioxaborin Put alkane (16.3mL, 80mmol) into a 250ml three-necked flask, dissolve it in 80mL toluene, stir and dissolve at room temperature in an argon atmosphere, and then raise the temperature to 80°C for 18h. Stop the reaction and pour the reaction solution into water to quench it. The reaction solution was concentrated and extracted with dichloromethane, purified by column chromatography, using 200-300 mesh silica gel as the stationary phase, and the eluent was petroleum ether / dichloromethane (2:1), to obtain 2-(3-( 3,10-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-14H-bis(dibenzothiophene)pyrrole-5-benzene Base-1,3,4-oxadiazole (10.1g, yield 70%), its chemical reaction equation is:
[0035]
[0036] Example 3
[0037] Add 2-aminobenzaldehyde (4.8g, 40mmol) and 50mL of acetone into a 250mL ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com