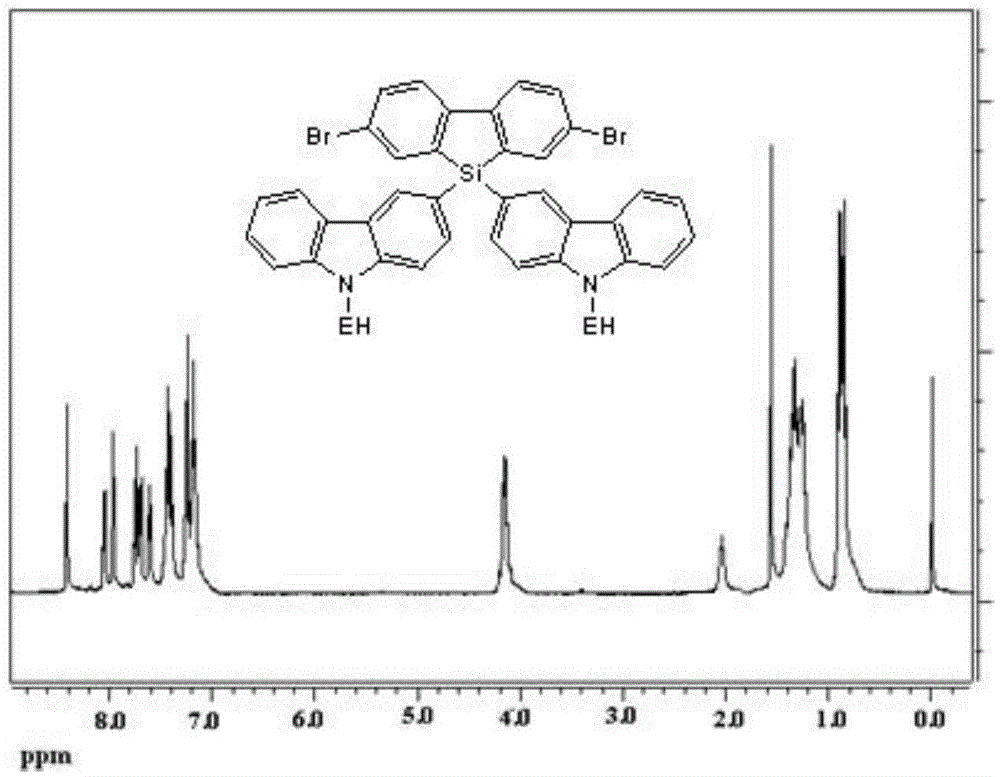
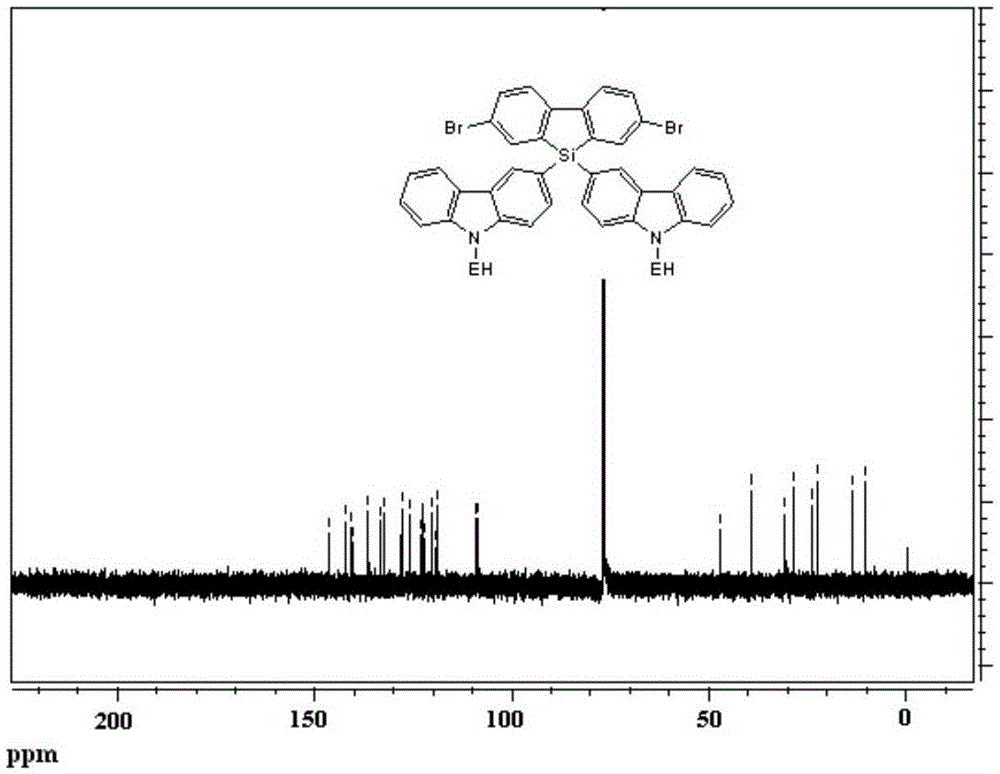
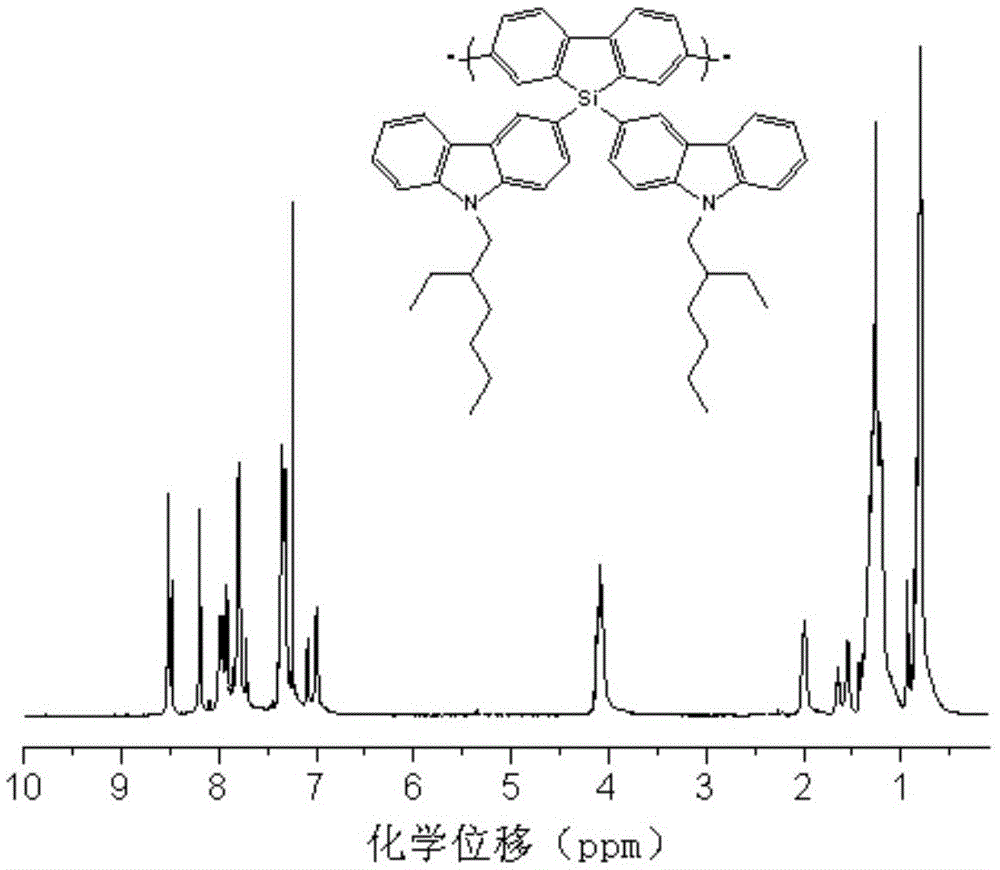
Polysilafluorene luminescent material with hole transmission groups, and preparation and application thereof
A technology of hole-transporting groups and light-emitting materials, which is applied in the field of polysilfluorene light-emitting materials and its preparation and application, can solve the problems of low efficiency of blue light-emitting materials, and achieve good hole injection and transport, good application prospects, The effect of high device efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of 2,2'-dinitro-4,4'-dibromobiphenyl
[0034] 140 grams of 2,5-dibromonitrobenzene, 120 grams of copper powder, and 500 ml of DMF were reacted at about 125 degrees for about 5 hours, cooled, and 400-500 ml of toluene was added, and the copper powder was filtered off. The filtrate was washed with copious amounts of water. Anhydrous magnesium sulfate was added to dry. Filter and spin off toluene. A yellow solid was obtained. Recrystallization from 100 ml of ethanol gave 75 g of a yellow powder with a yield of 75%. 1 HNMR (400MHz, CDCl 3 )δ7.18(2H,d,J=8.2),7.85(2H,dd,J=8.2,2.0),8.39(2H,d,J=2.0); 13 CNMR (100MHz, CDCl 3 ) δ122.9, 128.1, 131.9, 132.0, 136.6, 147.4.
[0035] (2) Preparation of 2,2'-diiodo-4,4'-dibromobiphenyl
[0036] Pre-frozen 10 / 20 g sodium nitrite / water solution and 100 / 240 g potassium iodide / water solution;
[0037] Place 20 grams of 2,2'-dinitro-4,4'-dibromobiphenyl obtained in step (1), 20 grams of iron powder, and about 600 mi...
Embodiment 2
[0046] (1) Synthesis of 4-(methoxydecyloxy-(4-bromobenzene))-triphenylamine
[0047] The synthetic route is shown below:
[0048]
[0049] The specific synthesis steps are: take a 250mL single-neck flask, add 4.94g (10mmol) 1-[4-[N-(diphenylamino)phenyl]methoxy]-10-bromodecane, 2g (11.56mmol) 4- Bromophenol, 2.76g (20mmol) potassium carbonate, 0.23g potassium iodide, DMSO 100mL. After changing nitrogen three times, it was heated to 132°C and the reaction was stirred overnight. The next day, extracted with toluene, washed with saturated brine, dried over magnesium sulfate, and purified by silica gel column chromatography. Colorless mucus, 76% yield. of the resulting product 1 H spectrum and 13 The C spectrum is as Figure 4 and Figure 5 shown. The identification data is as follows: 1 HNMR (400MHz, CDCl 3 ,ppm)7.256(d,2H,J=8.8),7.257~7.212(6H),7.10~7.05(6H),7.000(t,2H),6.770(d,2H,J=8.8),4.442(s, 2H), 3.910(t, 2H), 3.496(t, 2H), 1.765(m, 2H), 1.632(m, 2H), 1.40~1.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com