Application of LncRNAGAS5 to treatment on primary osteoporosis
A technology for osteoporosis and primary disease, applied in the field of molecular biomedicine, can solve the problems of decreased skin absorption, poor patient comfort, scar hyperplasia, etc., and achieve strong anti-osteoporosis ability, improved acceptance and comfort Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 constructs the adenovirus of GAS5 overexpression plasmid
[0022] The target shuttle plasmid pAdeno-mCMV-GAS5-EGFP-3FLAG was co-transfected with the adenovirus backbone plasmid into HEK293 cells to recombine to obtain the virus Adeno-GAS5-EGFP-3FLAG, which was purified after massive amplification, and the titer and target gene expression were detected Standby after leveling. The specific steps of the experiment are as follows:
[0023] 1. Virus packaging and identification
[0024] 1. The day before transfection, inoculate the cells into a 6-well plate, and control the density of the cells to be 70%-80% during transfection.
[0025] 2. Take out the cell culture plate one hour before transfection, take out the original cell culture medium, add 1.5ml of Opit-MEM medium, the total volume is 250ul, and mix gently.
[0026] 3. Prepare the complex of the transfection kit plasmid:
[0027] A. Dissolve 4ug of viral vector plasmid to be transfected (backbone pla...
Embodiment 2
[0062] Embodiment 2 animal experiments
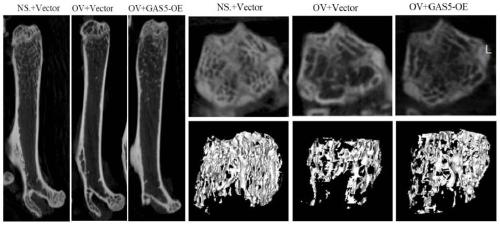
[0063] 1. Construction of primary osteoporosis mouse model
[0064] Thirty C57 female mice were divided into 3 groups, normal growth + control virus group; osteoporosis mouse model + control virus group; osteoporosis mouse model + GAS5 overexpression adenovirus group. At the age of 12 weeks, the latter two groups were subjected to ovariectomy, and their ovaries were removed to establish a mouse model of primary osteoporosis.
[0065] 2. GAS5 overexpression adenovirus and control were used for in vivo injection.
[0066] Two months later, the adenovirus containing the GAS5 overexpression plasmid was injected through the tail vein using a microsyringe. 5 x 10 per mouse 11 The virus particles were dissolved in 200ul saline and injected into the tail vein.
[0067] 3. Detection of bone formation indexes and bone quality of mice in each group
[0068] After February and May, blood was collected from eyeballs, serum was collected, and se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com