Preparation method of epoxy chloropropane
A technology of epichlorohydrin and chloropropene, applied in chemical instruments and methods, molecular sieve catalysts, organic chemistry, etc., can solve the problems of high energy consumption, difficult control of reaction temperature, and easy occurrence of side reactions in the saponification and separation process, and achieve side effects The effect of less reaction, easy separation of products, and reduction of potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
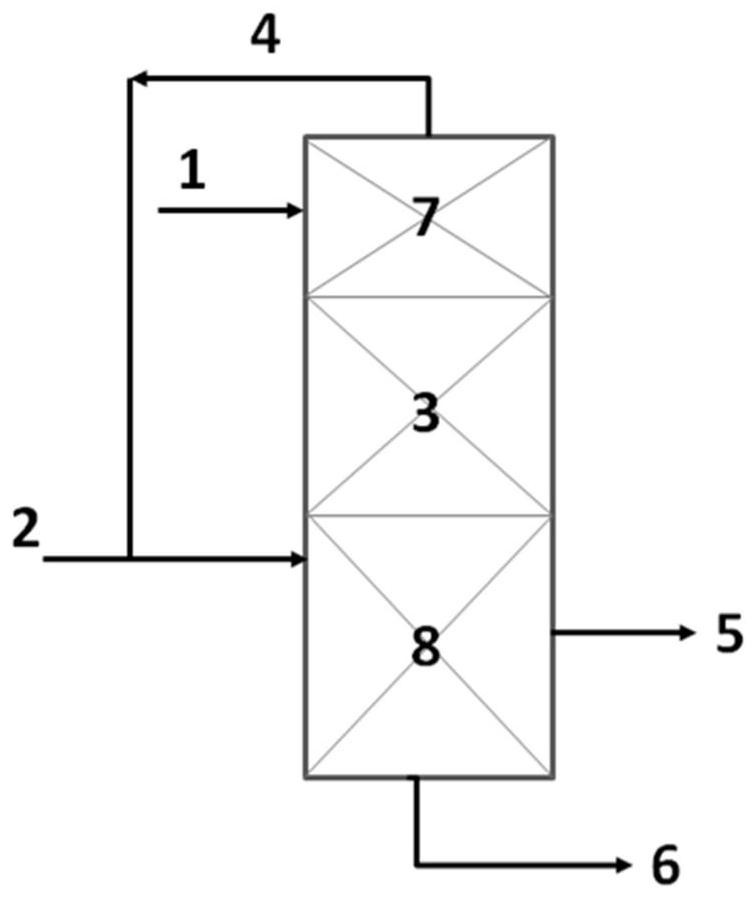
[0011] An embodiment of the present invention provides a method for preparing epichlorohydrin, which includes: using a fixed-bed catalytic reactor to reversely feed chloropropene and hydrogen peroxide, and using the balance of solvent vaporization and condensation to maintain the solvent at the level containing the catalyst In the reaction bed layer, allyl chloride and hydrogen peroxide react in the reaction bed layer to generate epichlorohydrin.
[0012] The use of fixed bed technology is an effective method to solve the difficult separation of catalysts in the slurry bed reaction process. It has the characteristics of easy solid-liquid separation, good operating flexibility, large production capacity and simple process. However, since the epoxidation process of allyl chloride is a strong exothermic reaction, it is difficult to control the reaction temperature when a fixed bed is used, resulting in more by-products and potential safety hazards.
[0013] The present invention ...
Embodiment 1
[0034] Embodiment 1 catalyst preparation
[0035] Put 95 grams of commercially available titanium-silicon molecular sieve TS-1 and 5 grams of silica sol on the forming agent to form a columnar catalyst with a diameter of 3 mm and a length of 3 mm, numbered CAT-1.
[0036] Put 80 grams of commercially available titanium-silicon molecular sieve Ti-MWW and 20 grams of aluminum sol on the molding agent to form a spherical catalyst with a diameter of 2.5 mm, numbered CAT-2.
[0037] 60 grams of titanium-silicon molecular sieve TS-2, 20 grams of silica sol and 20 grams of aluminum sol made by the patent CN1111092C modification method are used to form a trilobate-shaped catalyst with an equivalent diameter of 1 mm and a length of 2 mm on the molding agent, numbered CAT -3.
Embodiment 2
[0039] Put 5 grams of Catalyst CAT-1 into figure 1 In the reaction bed layer 3 of the shown reactor, separation fillers are respectively loaded at the upper end 7 and the lower end 8 of the reactor. Put an appropriate amount of solvent methanol into the reactor, and control the temperature in the middle of the reaction bed 3 to 60°C through the heat supply at the lower end 8 of the reactor and the heat extraction at the upper end 7, so that the solvent methanol reaches the equilibrium of vaporization and condensation, and stays at the Catalyst reaction bed 3. With hydrogen peroxide feed space velocity as 1h -1 , transporting 30% hydrogen peroxide to the reactor from the upper end of the reactor, and transporting allyl chloride to the reactor from the lower end of the reactor at the same time, the molar ratio of allyl chloride to hydrogen peroxide is 1:1, and carries out countercurrent epoxidation reaction.
[0040] Results: The conversion rate of hydrogen peroxide was 100%, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com