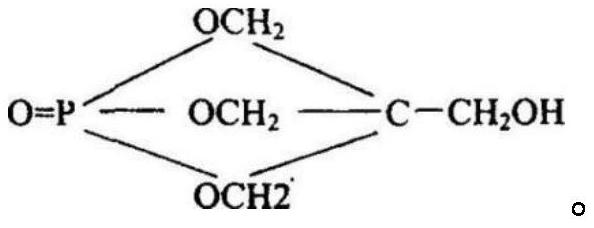
Preparation method of pentaerythritol diphosphate
A pentaerythritol and bisphosphonate technology is applied in the field of preparation of pentaerythritol-type bisphosphonates, and can solve the problems of easy decomposition of products, high reaction temperature, difficulty in industrialized large-scale production, etc., and achieves short reaction time, reduced reaction temperature, The effect of shortened time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The invention provides a kind of preparation method of pentaerythritol type diphosphate, comprises the following steps:
[0026] (1) under the action of a catalyst, phosphorus oxychloride and phenolic compounds are subjected to the first esterification reaction to obtain phosphoryl dichloride compounds;
[0027] (2) Under vacuum and decompression conditions, sequentially add phosphoryl dichloride compound and acid-binding agent to the pentaerythritol solution to carry out the second esterification reaction to obtain pentaerythritol-type bisphosphate.
[0028] In the present invention, under the action of a catalyst, phosphorus oxychloride and phenolic compounds are subjected to a first esterification reaction to obtain phosphorus oxydichloride compounds. In the present invention, the catalyst is preferably a Lewis acid; the Lewis acid is preferably one or more of titanium tetrachloride, magnesium chloride and aluminum chloride; the amount of the catalyst is preferably t...
Embodiment 1
[0045] Add 460kg (3.0kmol) of phosphorus oxychloride and 2kg of magnesium chloride into the reaction kettle and slowly raise the temperature to 80-85°C, start to add 94kg (1kmol) of phenol dropwise, and finish the dropwise addition in about 1 hour. Insulate for 2 hours, after the reaction is complete, lower the temperature to room temperature and slowly distill phosphorus oxychloride under reduced pressure. Collect the 110-120°C fraction under the condition of a temperature of 5-10kPa to obtain 190kg of phenoxyphosphoryl dichloride intermediate, yield: 90%, content: 98% (gas chromatography);
[0046] Add 61.3kg (0.45kmol) of pentaerythritol and 100kg of xylene into the reaction kettle, open the reflux condenser, the cooling temperature of the condenser is -5~0℃, in order to prevent the toluene from being taken away, add phosphoryl dichloride dropwise under reduced pressure Compound 190kg (0.9kmol), vacuum degree 70 ~ 75kPa, exothermic heat is obvious during the dropping proces...
Embodiment 2
[0048] Add 950kg (6.20kmol) of phosphorus oxychloride and 4kg of magnesium chloride into the reaction kettle and slowly raise the temperature to 80-85°C, start to add 190kg (2kmol) of phenol dropwise, and finish the dropwise addition in about 2 hours. Keep warm for 3 hours. After the reaction is complete, lower the temperature to room temperature and slowly distill phosphorus oxychloride under reduced pressure. The vacuum degree is 30-40kPa. Distill to 90-95°C to stop the distillation. The recovered phosphorus oxychloride is reused, and then the The 110-120°C fraction was collected under the condition of ~10kPa to obtain 386kg of phenoxyphosphoryl dichloride intermediate, yield: 91.5%, content: 98.2% (gas chromatography).
[0049] Add 130kg (0.95kmol) of pentaerythritol and 200kg of xylene into the reaction kettle, open the reflux condenser, the cooling temperature of the condenser is -5~0°C, to prevent the xylene from being taken away, add phosphoryl dichloride dropwise under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com