Preparation method and application of doxorubicin drug carrier La/Tm-MOFs@SiO2 composite material
A doxorubicin and carrier technology, which is applied in the preparation field of doxorubicin drug carrier La/Tm-MOFs@SiO2 composite material, can solve problems such as complexity, detection methods that cannot fully reflect drug delivery, and inability to provide accurate information in real time , to achieve the effects of enhanced energy transfer, low preparation cost, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
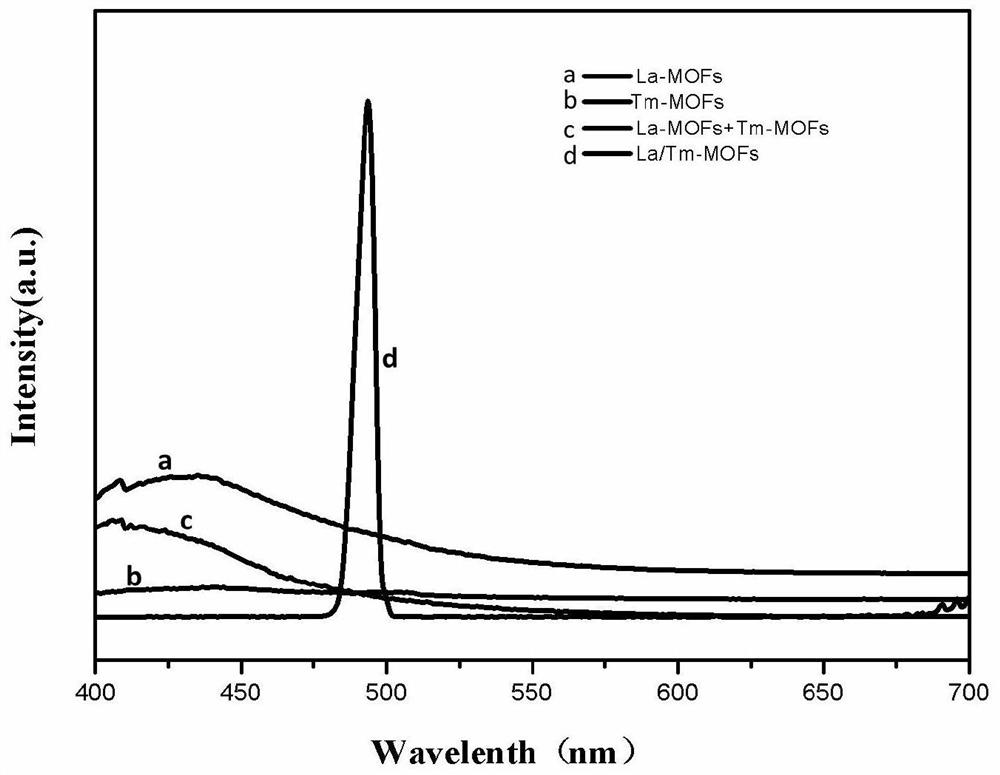
[0037] The preparation method of La-MOFs: Weigh 250mg of lanthanum nitrate and dissolve it in 20mL deionized water, weigh 250mg of trimesic acid and dissolve it in 20mL of absolute ethanol, mix the lanthanum nitrate solution and the trimesic acid solution to obtain a white solution, Then react at 140° C. for 24 hours, separate the precipitate by centrifuge, and repeatedly wash 3 times with deionized water and absolute ethanol (washing sequence: deionized water, absolute ethanol, deionized water) and centrifuge the precipitate. Then put it in an oven and dry it at 50°C to get La-MOFs material.
[0038] The preparation method of Tm-MOFs: Weigh 25 mg of thulium chloride and dissolve it in 20 mL of deionized water, weigh 250 mg of trimesic acid and dissolve it in 20 mL of absolute ethanol, mix the thulium chloride solution and trimesic acid solution to obtain white solution, then reacted at 140 ° C for 24 hours, separated the precipitate by a centrifuge, and repeatedly washed 3 ti...
Embodiment 1
[0041] An aminated silica-wrapped La / Tm-MOFs (La / Tm-MOFs@SiO 2 ) preparation method, comprising the following steps in turn:
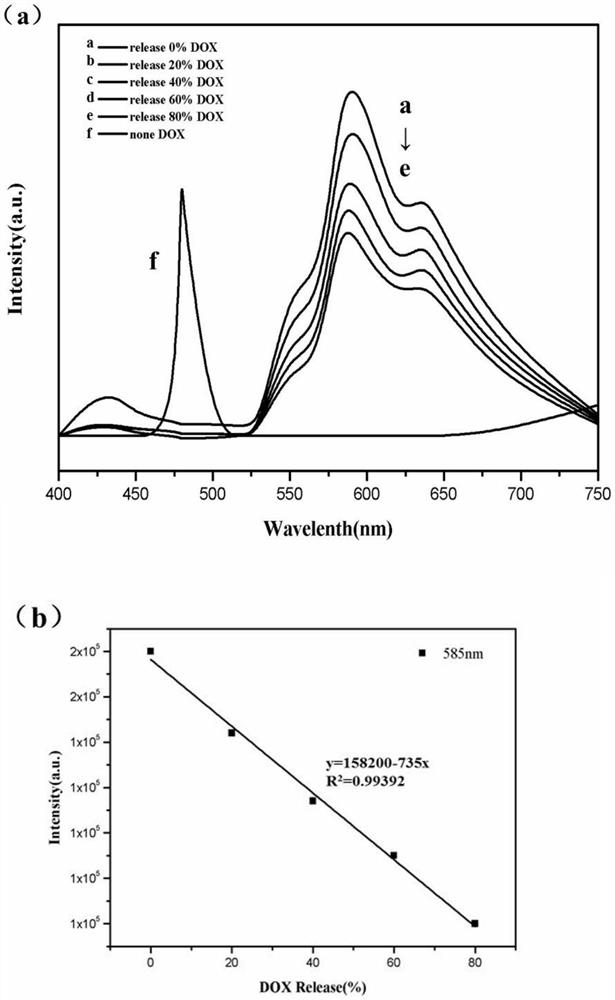
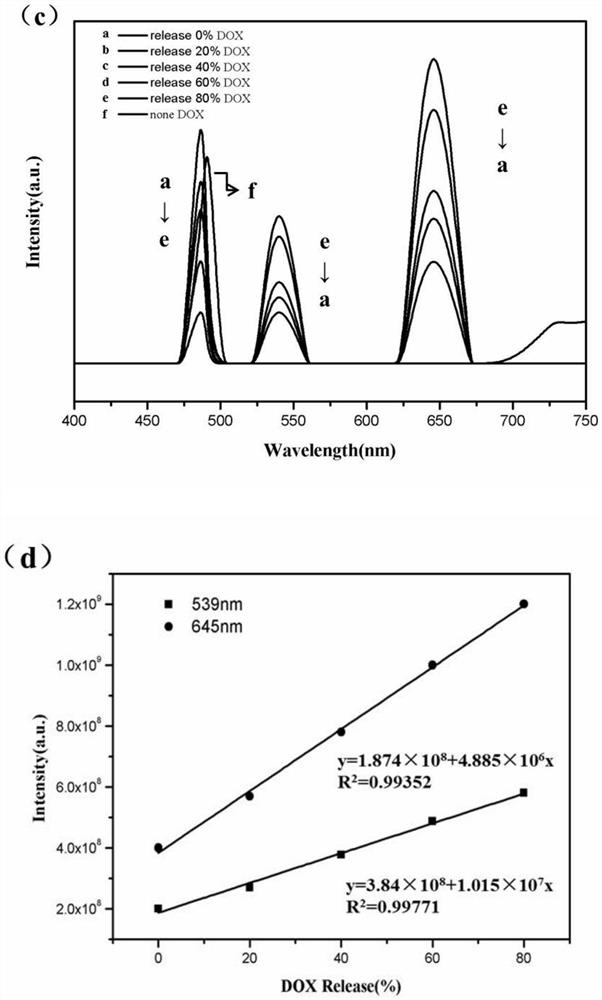
[0042] 1) Weigh 250 mg of lanthanum nitrate and 20 mg of thulium chloride and dissolve them in 20 mL of deionized water, weigh 250 mg of trimesic acid and dissolve them in 20 mL of absolute ethanol, and dissolve the lanthanum nitrate solution, thulium chloride solution, and trimesic acid solution in three Mix the two solutions to obtain a white solution, then react at 140°C for 24 hours, cool to room temperature, separate the precipitate by a centrifuge, and repeatedly wash 3 times with deionized water and absolute ethanol (washing order: deionized water, anhydrous Ethanol, deionized water, the following examples are the same, and will not be described in detail) and the precipitate is centrifuged. Then put it in an oven and dry it at 60°C to get La / Tm-MOFs material.
[0043] 2) Dissolve 400mg of cetyltrimethylammonium bromide and 150mg of La / Tm-MOFs...
Embodiment 2
[0049] An aminated silica-wrapped La / Tm-MOFs (La / Tm-MOFs@SiO 2 ) preparation method, comprising the following steps in turn:
[0050] 1) Weigh 270 mg of lanthanum nitrate and 18 mg of thulium chloride and dissolve them in 22 mL of deionized water, weigh 225 mg of trimesic acid and dissolve them in 15 mL of absolute ethanol, and dissolve the lanthanum nitrate solution, thulium chloride solution, and trimesic acid solution in three The solution was mixed to obtain a white solution, and then reacted at 140°C for 24 hours, cooled to room temperature, and the precipitate was separated by a centrifuge, and the precipitate was repeatedly washed 3 times with deionized water and absolute ethanol and centrifuged. Then put it in an oven and dry it at 60°C to get La / Tm-MOFs material.
[0051] 2) Dissolve 380 mg of cetyltrimethylammonium bromide and 175 mg of La / Tm-MOFs prepared in step (1) in 18 mL of absolute ethanol. After ultrasonic dissolution, add 70 mL of deionized water, 1.5 mL of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com