Anti-African swine fever virus fusion protein as well as preparation method and application thereof
A fusion protein and anti-virus technology, applied in the field of genetic engineering, can solve the problems that antibiotic veterinary drugs cannot be cured and controlled, cannot enter the blood circulation system, and lose active functions. It takes a long time to achieve drug efficacy, good drug stability, and Effect with low side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Amplification of DN-α fragments:
[0040] (1) Using the synthetic IFN-α whole gene sequence (shown in SEQ ID No.9) as a template, use the following primers to perform PCR amplification to obtain the IFN-α fragment:
[0041] TGCGATCTGCCGCAGACCCATAGCCTGGCGCATACCCGCGCGCTGCGCCTGCTGGCGCAGATGCGCCGCATTAGCCCGTTTAGCTGCCTGGATCATCGCCGCGATTTTGGCTTTCCGCAGGAAGCGCTGGGCGGCAACCAGGTGCAGAAAGCGCAGGCGATGGCGCTGGTGCATGAAATGCTGCAGCAGACCTTTCAGCTGTTTAGCACCGAAGGCAGCGCGGCGGCGTGGGATGAAAGCCTGCTGCATCAGTTTTGCACCGGCCTGGATCAGCAGCTGCGCGATCTGGAAGCGTGCGTGATGCAGGAAGCGGGCCTGGAAGGCACCCCGCTGCTGGAAGAAGATAGCATTCTGGCGGTGCGCAAATATTTTCATCGCCTGACCCTGTATCTGCAGGAAAAAAGCTATAGCCCGTGCGCGTGGGAAATTGTGCGCGCGGAAGTGATGCGCGCGTTTAGCAGCAGCCGCAACCTG(SEQ ID No.9)
[0042] PCR amplification system: PFU ultra II Fusion buffer 2μl, dNTP (10mM each) 0.4μl, FWprimer (20μM) 0.4μl, REV primer (20μM) 0.4μl, Template 0.1μl, Sterile miliQ water16.3μl, pFU ultra IIfusion enzyme 0.4 μl.
[0043] The PCR reaction procedure is as follows:
[...
Embodiment 3
[0101] For verifying beneficial effect of the present invention, carry out following test, test method and result are as follows:
[0102] (1) Experimental animals
[0103] A farm has a group of pigs, 1-5 parity-age sows, weighing 50-200kg, some of them have symptoms such as high fever, dyspnea, cough, runny nose, skin cyanosis, anorexia, depression, and sow abortion. Various antibiotics are used And antipyretic peony has no therapeutic effect, and a large number of deaths in a short period of time. Symptoms and autopsy confirmed infection with African swine fever.
[0104] (2) Clinical trials
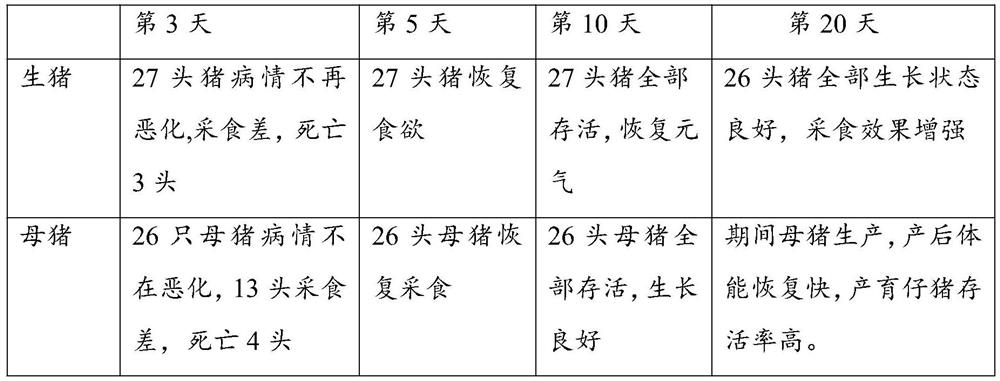
[0105] Mix the prepared yeast cells containing fusion proteins DN-α and DN-γ at a mass ratio of 1:1; then add 100g of dry yeast and 100g of Bacillus for every 1g of the mixture, and mix them evenly, and randomly select them on the diseased pig farm 30 sick pigs were used as the administration group, and the pigs were fed with 1 g per 100 kg of pig body weight for 10 consecutive days...
Embodiment 4
[0112] Drugs for the prevention and / or treatment of African swine fever disease
[0113] The prepared yeast cells containing the fusion proteins DN-α and DN-γ were mixed at a mass ratio of 1:1; then, according to every 1g of the mixture, 100g of dry yeast was added, and 100g of bacillus was mixed evenly to obtain the drug. The daily feeding amount of a pig is 1g, and 10 consecutive days of feeding is a course of treatment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com