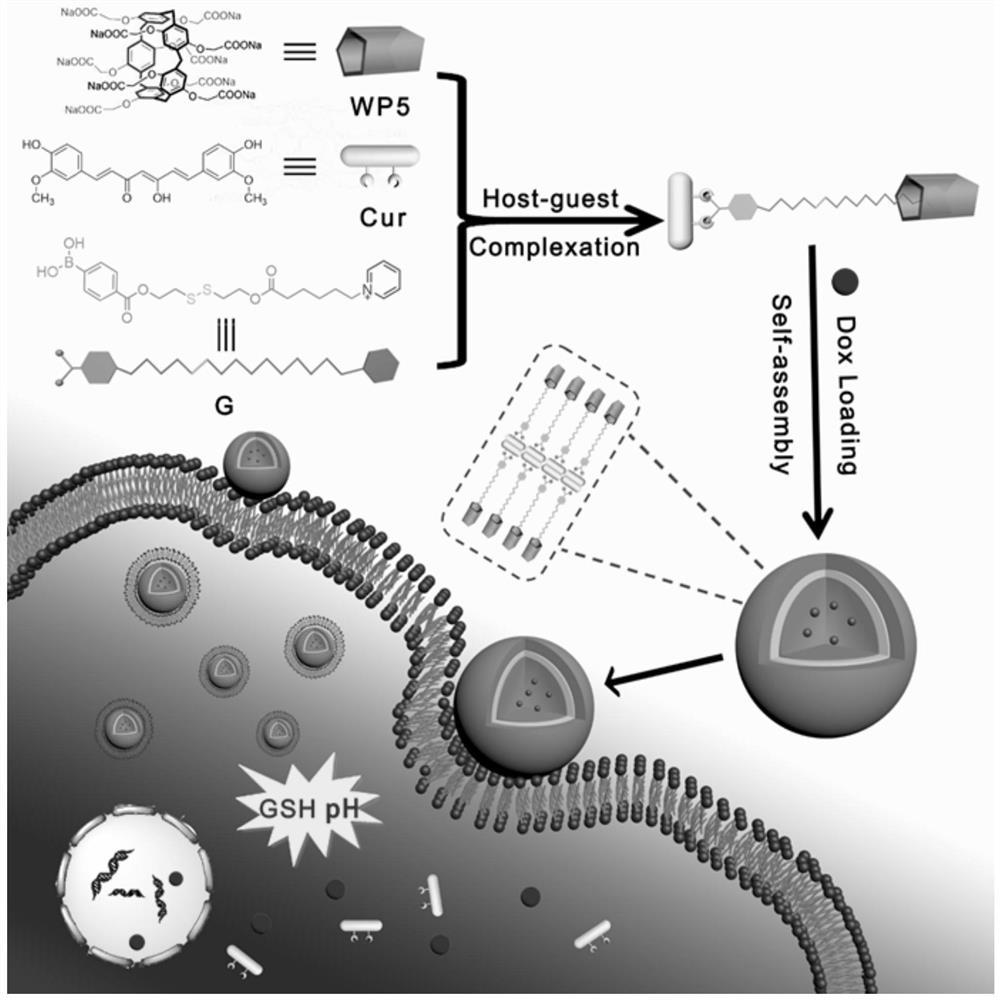
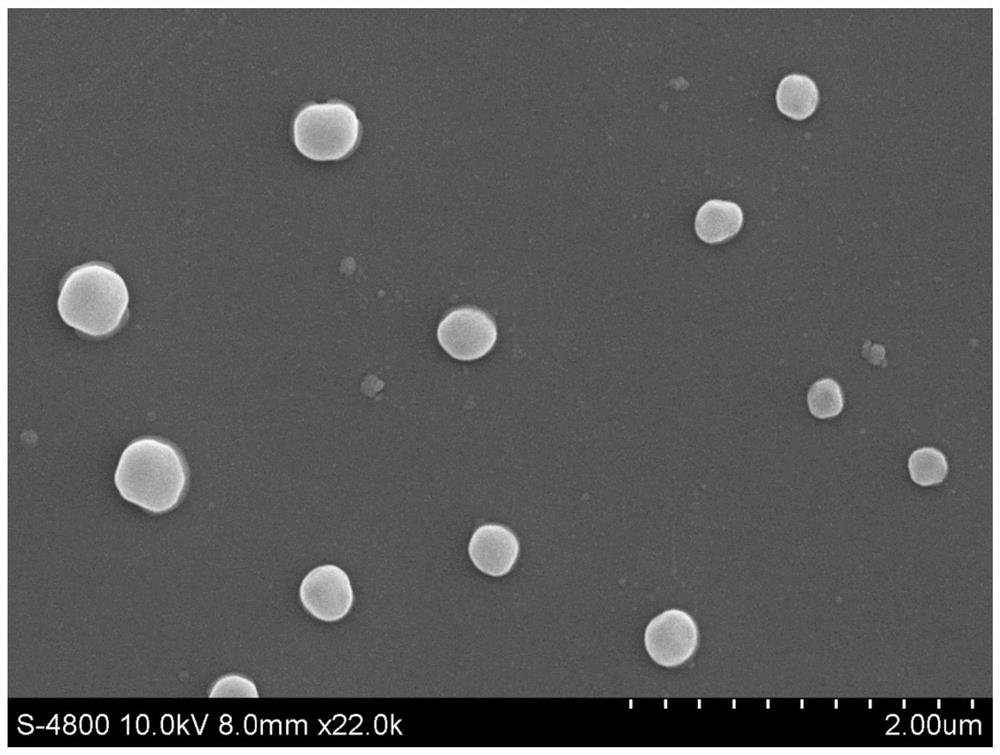
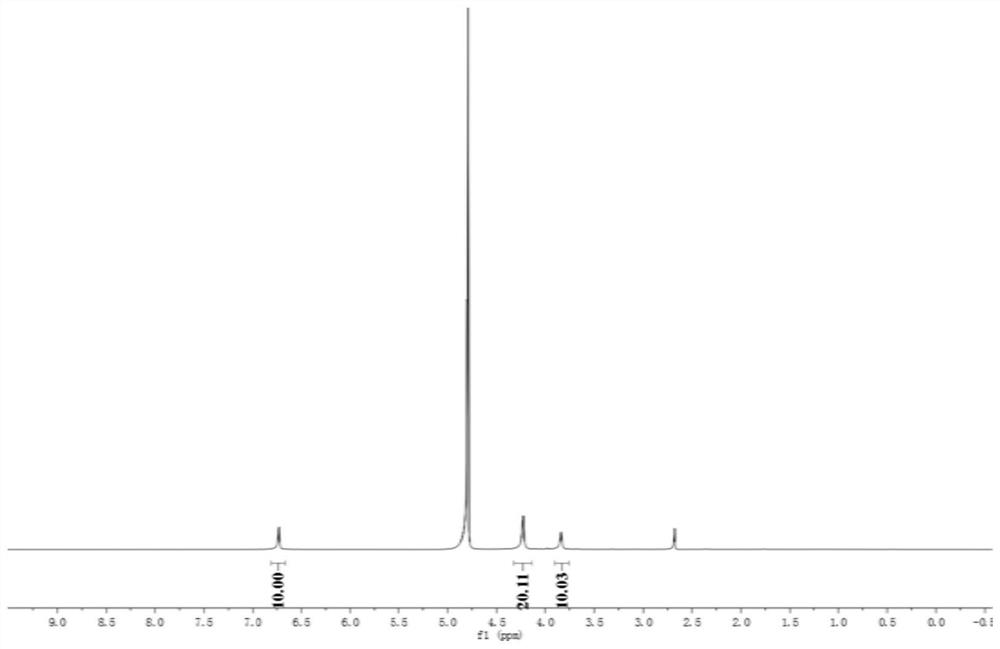
A ph-gsh dual-responsive nano-curcumin prodrug loading system
A curcumin-based, responsive technology, applied in the field of nano-biomedical materials, can solve the problems of low drug release rate, complex system structure, and single stimulus response, and achieve good application and development prospects, high drug loading, and improved water solubility. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Synthesis of Compound 2
[0046] The synthetic route of compound 2 is as follows:
[0047]
[0048] Accurately weigh 1,4-dimethoxybenzene (0.691 g, 5 mmol) and paraformaldehyde (0.450 g, 15 mmol) into a 100 mL round-bottomed flask, and add 60 mL of 1,2-dichloroethane to dissolve. Then, boron trifluoride ether (0.75 ml, 6 mmol) was added to the above solution, and the mixture was stirred at room temperature for 2 h under nitrogen protection. After the reaction was completed, ice water was added to quench the reaction. Filter at atmospheric pressure, then use a rotary evaporator to remove the solvent. The residue was dissolved in dichloromethane (DCM), washed 3 times with deionized water, saturated NaHCO solution and saturated NaCl solution, respectively, the organic phase was collected and dried with anhydrous MgSO for 2 h, filtered, and the organic solvent was removed by distillation under reduced pressure, The crude product can be obtained and separated...
Embodiment 2
[0049] Example 2: Synthesis of Compound 3
[0050] The synthetic route of compound 3 is as follows:
[0051]
[0052] Accurately weigh compound 2 (0.660 g, 0.88 mmol) into a 50 mL round-bottomed flask, add 20 mL of dry DCM to dissolve, add boron tribromide (4 mL, 43 mmol) dropwise, and stir at room temperature for 24 h under nitrogen protection. After the reaction was completed, ice water was added to quench the reaction. After adding ice water and stirring for 30 min, the white solid was obtained by suction filtration and washed with 1 mmol / L hydrochloric acid and chloroform to obtain compound 3. (The compound is not stable, after selection, proceed directly to the next step).
Embodiment 3
[0053] Example 3: Synthesis of Compound 4
[0054] The synthetic route of compound 4 is as follows:
[0055]
[0056] Compound 3 (0.610 g, 1 mmol) was dispersed in 30 mL of acetonitrile, and K was added to the suspension 2 CO3 (1.658 g, 12 mmol) was stirred at room temperature for 30 min. Then, ethyl bromoacetate (2 mL, 18 mmol) and KI (0.166 g, 1 mmol) were added, and the mixture was refluxed for 24 h under nitrogen protection. After the reaction is over, the solution is cooled naturally and filtered to remove excess K 2 CO3, then the solvent was removed using a rotary evaporator. The residue was redissolved in DCM, washed three times with deionized water and saturated NaCl solution, respectively, the organic phase was collected and washed with anhydrous Na 2 SO4 dried for 2h. After filtration at normal pressure, after the organic solvent was distilled off under reduced pressure, the crude product was obtained, and the orange solid 4 was obtained by column chromatogra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com