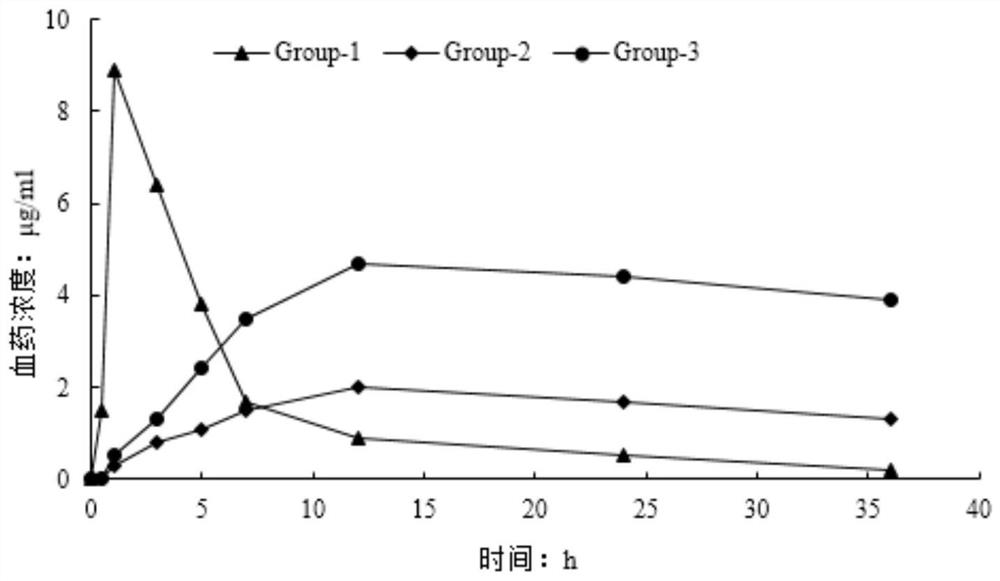
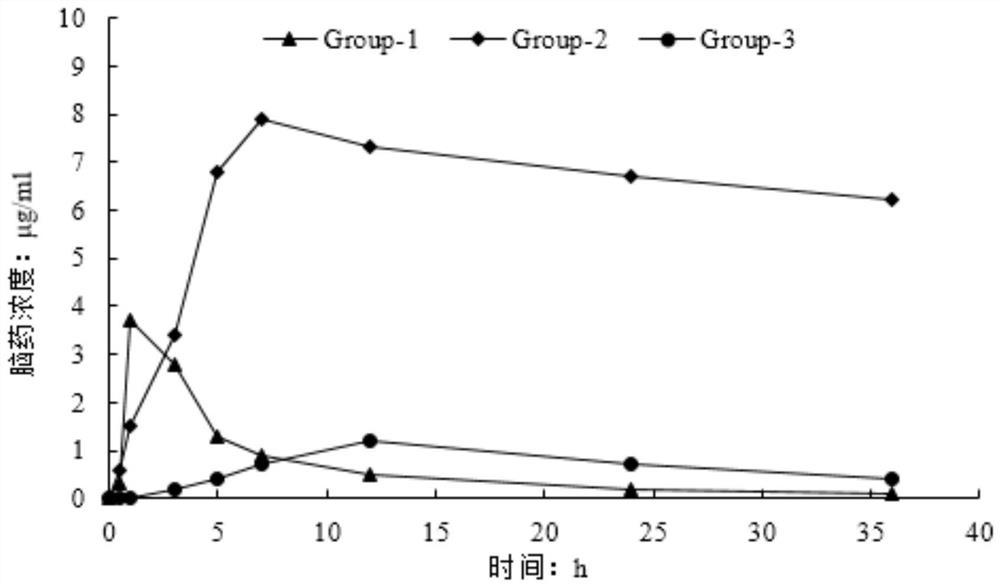
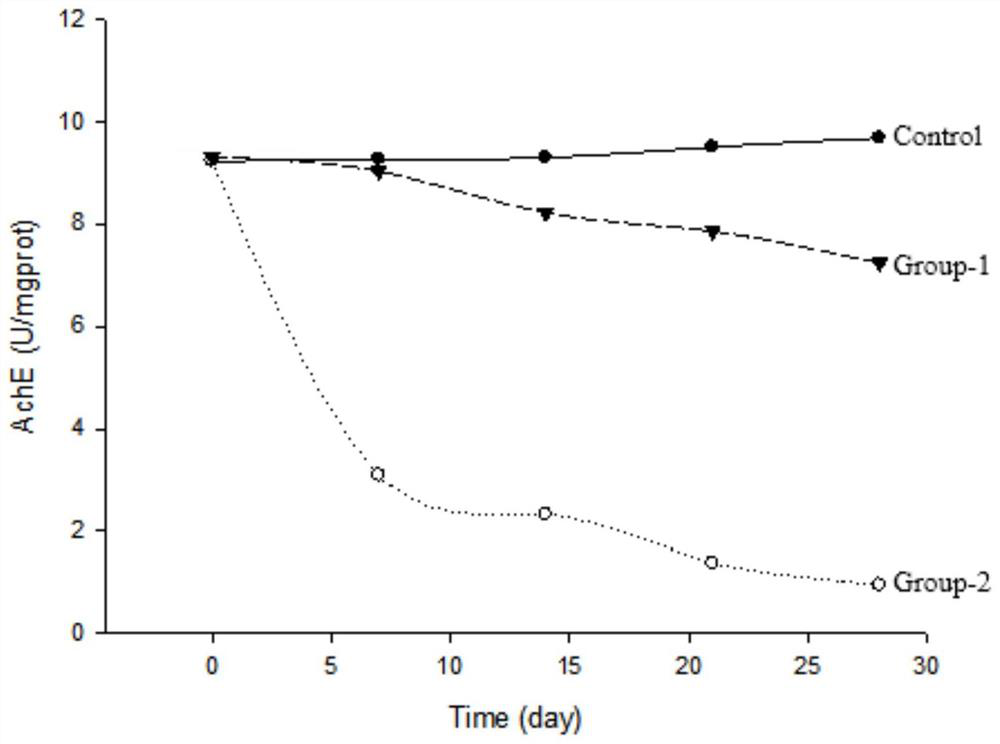
Huperzine A acupoint sustained-release gel patch for treating senile dementia and preparation method thereof
A slow-release gel patch and huperzine A technology, which is applied in the field of medicine, can solve the problems that patients cannot take medication continuously, and achieve the effects of long drug action time, large drug loading capacity, and high drug transdermal absorption efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The above-mentioned preparation method of huperzine A acupoint sustained-release gel patch for treating senile dementia, huperzine A is prepared by fermentation and biosynthesis of the following engineering bacteria, and the engineering bacteria used include: Mucoris racemosa NSH- D strain Mucorracemosus NSH-D (GenBank accession number KX060585.1), Mucor fragilis NSY-1 strain Mucor fragilis NSY-1 (GenBank accession number KX060586.1), Fusarium verticillioides NSH-5 strain Fusarium verticillioides NSH-5 (GenBank accession number KX853851.1), Fusarium oxysporum Fusarium verticillioides NSG-1 (GenBank accession number KX853850.1), Trichoderma harzianum NSW-V strain Trichoderma harzianum NSW-V (GenBank accession number KX034080.1), any of them One or more are refined through the steps of culture and fermentation, ultrasonic crushing of cells, extraction, concentration, purification and drying.
[0048] The key points of the present invention are described below from the pri...
Embodiment 1
[0053] Step 1: According to the requirements of the preparation, according to the mass percentage, weigh 0.0083% huperzine A, 2.7546% borneol, 0.6121% sodium polyacrylate NP-800, 1.3517% sodium polyacrylate NP-700, 2.4485% Titanium dioxide, 0.1530% aluminum glyoxate, 0.0918% EDTA-2Na, 35.1404% glycerin, 3.6728% PVP-K120, 0.1530% tartaric acid, 47.3089% purified water, 0.1836% preservatives (ethylparaben and Butylparaben 4:1), 6.1213% of 95% ethanol, the sum of the mass percentages of each component is 100%;
[0054] Step 2: Shake and mix the sodium polyacrylate NP-800, sodium polyacrylate NP-700, titanium dioxide, aluminum glycolate and EDTA-2Na weighed in step 1 in a sealed, clean and dry plastic bag to obtain a solid phase for later use;
[0055] Step 3: Add the glycerin weighed in step 1 into a vacuum mixer, then add the solid phase obtained in step 2, and then stir for 10 minutes under the condition that the vacuum degree is not lower than -0.05MPa to obtain the glycerin p...
Embodiment 2
[0061] Step 1: According to the requirements of the preparation, according to the mass percentage, weigh 0.0091% huperzine A, 4.3929% borneol, 1.0138% sodium polyacrylate NP-800, 0.9182% sodium polyacrylate NP-700, 3.3792% Titanium dioxide, 0.2365% aluminum glycylate, 0.1690% EDTA-2Na, 38.0966% glycerin, 4.7309% PVP-K90, 0.2365% tartaric acid, 39.7886% purified water, 0.2703% preservatives (ethylparaben and Butylparaben 4:1), 6.7584% of 95% ethanol, the sum of the mass percentages of each component is 100%;
[0062] Step 2: Shake and mix the sodium polyacrylate NP-800, sodium polyacrylate NP-700, titanium dioxide, aluminum glycolate and EDTA-2Na weighed in step 1 in a sealed, clean and dry plastic bag to obtain a solid phase for later use;
[0063] Step 3: Add the glycerin weighed in step 1 into a vacuum mixer, then add the solid phase obtained in step 2, and then stir for 10 minutes under the condition that the vacuum degree is not lower than -0.05MPa to obtain the glycerin p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com