Preparation method of nano drug carrier for treating cancer bone metastasis
A nano-drug carrier and bone metastasis technology, applied in cancer treatment technology and its application fields, can solve the problems of limited surgical treatment, limited effect of chemotherapy treatment, weak permeability, etc., and achieve the effect of overcoming limitations and synergistically killing tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Step 1: Dissolve 1 g of albumin in 100 ml of DMEM medium, wherein the concentration of phosphate is 1 mM, stir for 30 min, add 1 ml of calcium chloride (1 M) solution, which contains 1 mg of siRNA, continue stirring for 30 min, and then centrifuge , rotating speed 15000rpm, time 15min, add deionized water to wash twice, disperse in 10mL deionized water, it is CaP dispersion liquid;
[0022] Step 2: Dissolve 0.1 g of 1,2-distearoylphosphatidylethanolamine, 0.01 g of cholesterol and 0.01 g of docetaxel in chloroform, remove the chloroform by rotary evaporation, add 5 mL of the CaP dispersion in step 1, Shake on a shaker for 1h. Then add 0.2 g of alendronate sodium therein, react for 1 h, then add 1 mL of Tris buffer to terminate the reaction, centrifuge to remove unbound alendronate sodium, and obtain a nano drug carrier dispersion liquid bound to alendronate sodium.
Embodiment 2
[0024] Step 1: Dissolve 1 g of sodium hyaluronate in 100 ml of HEPES buffer (pH 7.4), stir for 30 min, add 1 ml of calcium chloride (1M) solution containing 1 mg of siRNA, continue stirring for 30 min, and then centrifuge. The rotation speed is 15000rpm, the time is 15min, and deionized water is added to wash twice, and dispersed in 10mL deionized water to form a CaP dispersion;
[0025] Step 2: Dissolve 0.1 g of 1,2-distearoylphosphatidylethanolamine, 0.01 g of cholesterol and 0.01 g of docetaxel in chloroform, remove the chloroform by rotary evaporation, add 5 mL of the CaP dispersion in step 1, Shake on a shaker for 1h. Then add 0.2 g of alendronate sodium therein, react for 1 h, then add 1 mL of Tris buffer to terminate the reaction, centrifuge to remove unbound alendronate sodium, and obtain a nano drug carrier dispersion liquid bound to alendronate sodium.
Embodiment 3
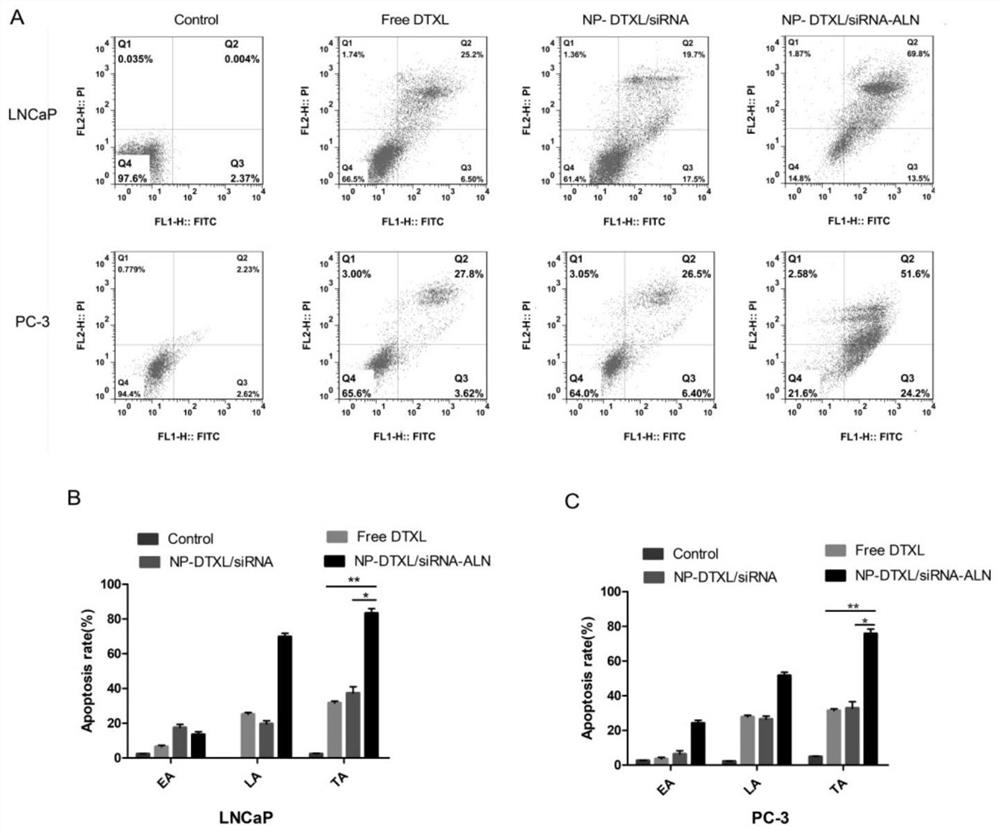
[0027]The PC-3 cells and LNCaP cells were divided into groups, and were divided into no drugs, adding docetaxel, drug-loaded nano-drug carriers and nano-drug carriers combined with alendronate sodium for treatment, and took out the culture plate after incubation for 24 hours . Aspirate the culture medium in the 6-well plate into a suitable centrifuge tube, wash the cells once with PBS, add 0.5mL of trypsin to digest the cells, digest for 2min, absorb the trypsin to avoid over-digestion of the trypsin, and add serum-free DMEM to culture Collect the cells with the culture medium collected above, and mix them together with the culture medium collected above, centrifuge (1000rpm×5min), discard the supernatant, add PBS to resuspend the cells, centrifuge again (1000rpm×5min) to remove the supernatant, add 195 μL of Annexin V- Resuspend the cells in FITC binding solution, then add 5 μL of Annexin V-FITC staining solution, mix gently, then add 10 μL of propidium iodide staining soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com