Kit and method for detecting related substances of genetic metabolic diseases by non-derivative tandem mass spectrometry
A technology for genetic and metabolic diseases and related substances, applied in measurement devices, instruments, scientific instruments, etc., can solve the problems of the influence of the test person and the limited types of diseases, and achieve the effect of increasing the variety, meeting the needs of use, and improving the needs of use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
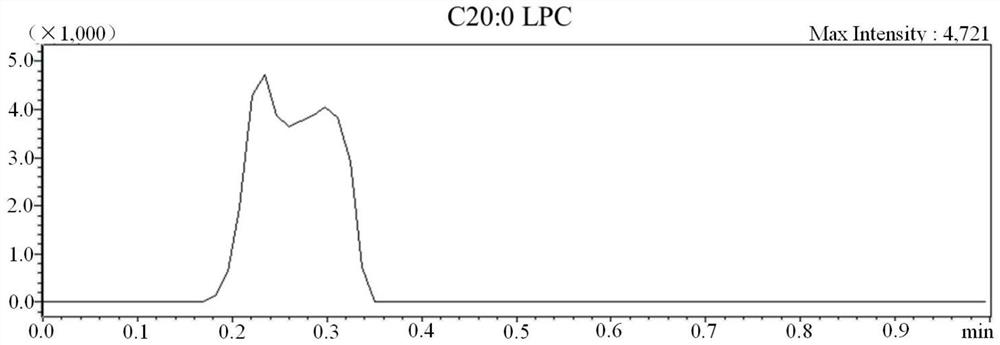
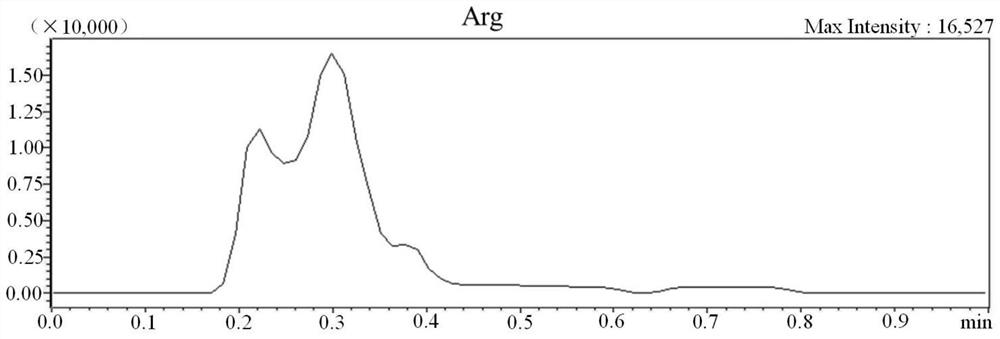
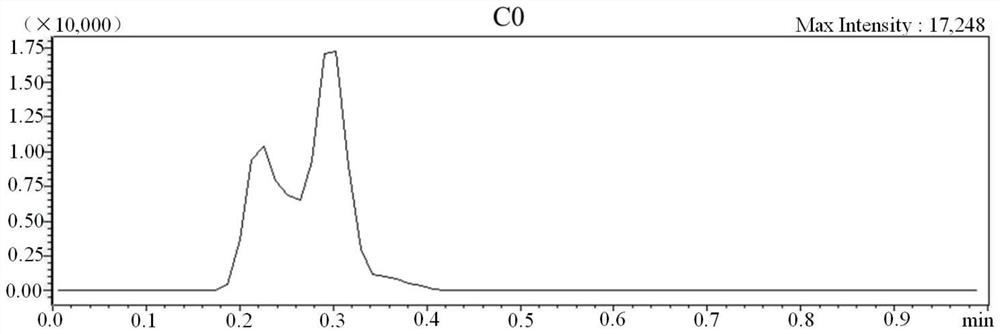
[0064] 1. Kit for detection of substances related to genetic metabolic diseases by non-derivatization method tandem mass spectrometry
[0065] The kit in this example is composed of two parts, namely A box and B box, A box is used for non-liquid parts, and B box is used for solutions, as shown in Table 5.
[0066] Table 5 Kit Components
[0067]
[0068]
[0069] Among them, the mixed isotope internal standard includes 13 kinds of amino acids, 14 kinds of carnitine, 1 kind of ketone, 1 kind of lysophosphatidylcholine, 2 kinds of acids and 2 kinds of adenosine, a total of 33 kinds of isotopic internal standards; the quality control substance is Dried blood spot samples containing 33 quality control analytes with known concentrations; 59 genetic metabolic disease-related substances, isotope standards and quality control analytes are shown in Table 1.
[0070] Table 1 59 substances related to genetic metabolic diseases and their isotope standards and quality controls
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com