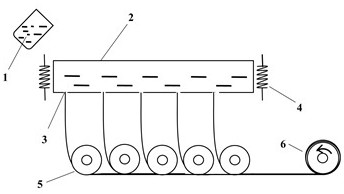
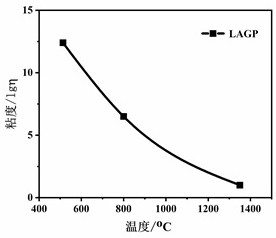
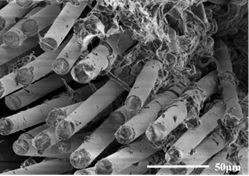
All-solid-state composite electrolyte based on glass fiber vertical array structure and preparation method thereof
A composite electrolyte and array structure technology, applied in the field of solid electrolyte materials, can solve problems such as ionic conductivity and safety limitations, and achieve the effects of low cost, enhanced lithium ion transport channels, and high ionic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Prepare polyethylene oxide (PEO, molecular weight 60W) and lithium bistrifluoromethanesulfonylimide (LiTFSI) at a molar ratio of 15:1 and mix them thoroughly.
[0042] (2) The solid electrolyte material of this example is Li 1.5 Al 0.5 Ge 1.5 (PO 4 ) 3 According to the stoichiometric ratio, 500g of lithium carbonate, alumina, germanium dioxide, and ammonium dihydrogen phosphate powder were weighed and mixed for ball milling. An additional 5wt% of lithium carbonate was added to compensate for the volatilization of lithium sources during high-temperature melting. After fully mixing, put the mixed powder in a corundum crucible, and heat it in a high-temperature electric melting furnace at 1350 o C for 30 minutes, until the formation of clarified molten glass, the molten glass is transferred to the platinum alloy slot-type drain plate heated by high-temperature electric fusion electrodes, and the temperature in the drain plate is controlled at 1250 ° c. It can be ...
Embodiment 2
[0048] (1) Polyvinylidene fluoride (PVDF, molecular weight 100W) and lithium hexafluorophosphate LiPF 6 Prepare according to the molar ratio of 25:1 and mix thoroughly.
[0049] (2) The solid electrolyte material of this example is Li 1.5 Al 0.5 Ti 1.5 (PO 4 ) 3 According to the stoichiometric ratio, 500g of lithium carbonate, alumina, titanium dioxide, and ammonium dihydrogen phosphate powder with a total mass of 500g were weighed and ball-milled, and an additional 5wt% of lithium carbonate was added to compensate for the volatilization of lithium sources during high-temperature melting. After fully mixing, the mixed powder was placed in an alumina crucible, at 1400 o Keep warm in the high temperature furnace of C for 30 minutes to clarify the molten state, then transfer the molten glass to the platinum alloy grooved bushing, and the temperature inside the platinum alloy grooved bushing is controlled at 1300 o c. The molten glass flows out along the nozzle at the botto...
Embodiment 3
[0053] (1) Polyethylene glycol (PEG, molecular weight 10000) and lithium hexafluorophosphate LiPF 6 Prepare according to the molar ratio of 25:1 and mix thoroughly.
[0054] (2) The solid electrolyte material of this example is Li 1.5 Al 0.5 Ti 1.5 (PO 4 ) 3 According to the stoichiometric ratio, 500g of lithium carbonate, alumina, germanium dioxide, and ammonium dihydrogen phosphate powder were mixed and ball milled, and an additional 5wt% of lithium carbonate was added to compensate for the volatilization of lithium sources during high-temperature melting. After fully mixing, put the mixed powder in a corundum crucible, and heat it in a high-temperature electric melting furnace at 1350 o C heat preservation for 30 minutes, until the clarified molten glass is formed, the molten glass is transferred to the platinum alloy grooved bushing, and the temperature inside the platinum alloy grooved bushing is controlled at 1250 °s c. The molten glass flows out along the nozzle ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com