Surface-modified perovskite catalyst as well as preparation method and application thereof
A surface modification and perovskite technology, applied in chemical instruments and methods, physical/chemical process catalysts, heterogeneous catalyst chemical elements, etc., can solve problems such as low specific surface area and single surface state of perovskite
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. carry out proportioning according to the chemical formula of perovskite catalyst, take by weighing 2.165g lanthanum nitrate (La(NO 3 ) 3 ·6H 2 O), 1.36785g cobalt nitrate (Co(NO 3 ) 3 ·6H 2 O), 0.44g platinum nitrate (Pt(NO 3 ) 2 ) was dissolved in 50mL of distilled water, and magnetically stirred to form a 0.1M solution.
[0031] 2. Add 2.9224g of ethylenediaminetetraacetic acid, which is equimolar to the total metal ions, and 4.2028g of citric acid with a molar ratio of 1:2 to the total metal ions, and keep stirring the above solution until it dissolves.
[0032] 3. Adjust the pH value of the above solution to 6 with ammonia water.
[0033] 4. The solution was kept in a water bath at 80°C for 8 hours to form a wet gel.
[0034] 5. Place the obtained wet gel in an oven to raise the temperature to 120°C at a rate of 5°C / min, keep it warm for 24 hours to form a dry gel, then transfer it to a muffle furnace, and raise the temperature to 400°C at a rate of 10°C / mi...
Embodiment 2
[0036] 1. Get the catalyst 0.15g prepared by Example 1 and place it in a beaker, add 30% H 2 o 2 15mL, then heated at 60°C for 6h to remove the moisture, and then dried at 110°C for 2h to obtain a surface-modified perovskite catalyst.
[0037] 2. Repeat the above steps twice to obtain the final sample.
Embodiment 3
[0039] 1. Get the catalyst 0.15g prepared by Example 1 and place it in a beaker, add 30% H 2 o 2 15mL, then heated at 60°C for 6h to remove the moisture, and then dried at 110°C for 2h to obtain a surface-modified perovskite catalyst.
[0040] 2. Repeat the above steps 4 times to obtain the final sample.
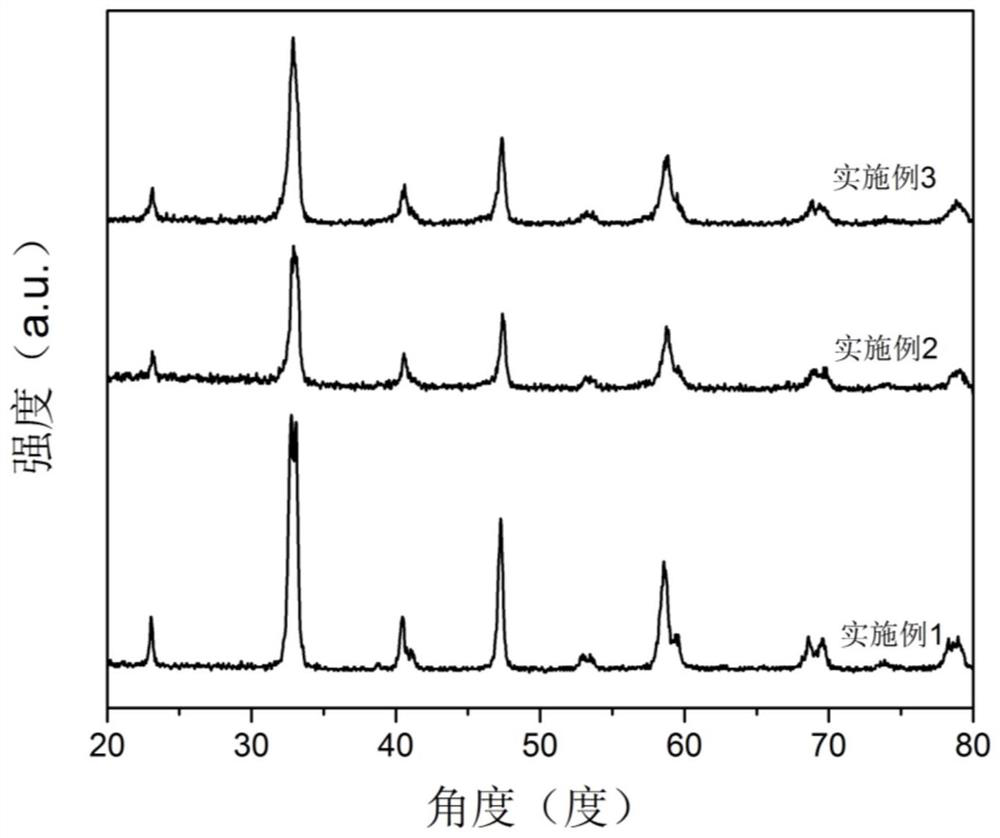
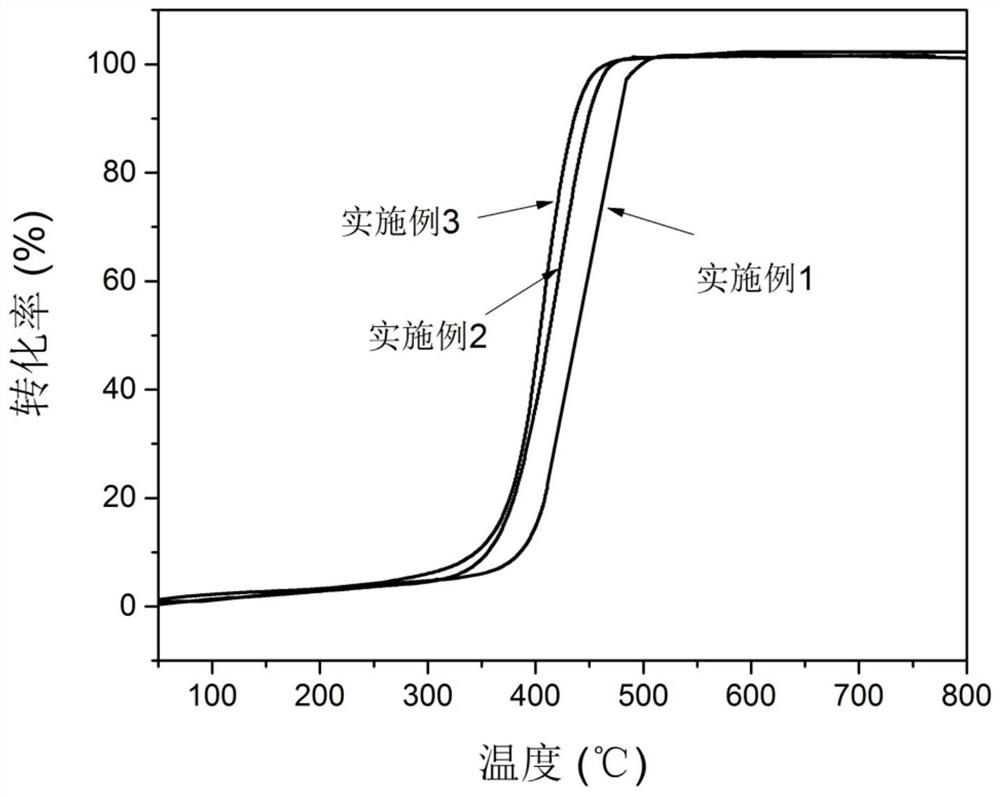
[0041] The catalyst prepared by the present invention is characterized, as attached figure 1 As shown, through the comparison of PDF cards, it was found that the perovskite catalyst was successfully prepared in Example 1, and it was found that after 30wt% H 2 o 2 (Hydrogen peroxide aqueous solution) treatment did not change the structure of the catalyst, and still maintained the perovskite structure. At the same time, it is not difficult to find that the crystallinity of the sample modified by hydrogen peroxide decreases, and the spatial structure of the perovskite gradually transforms from the trigonal crystal system to the cubic crystal system. From the perspective o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com