Myositis and myasthenia gravis autoantibody detection kit and method in human body fluid
A myasthenia gravis and detection kit technology, which is applied in the field of biomedicine, can solve the problems of time-consuming detection process, large demand for antibodies, and low detection sensitivity, and achieve the effects of short detection cycle, reduced pollution, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0092] The target antigen Ag in the detection kit of the present invention can be obtained through the existing technology, and can also be obtained through the following methods of the present invention, preferably the preparation method of the present invention. Specifically, the preparation method of myositis and myasthenia gravis autoantibody detection material in human body fluid of the present invention comprises the following steps:
[0093] Step 1, obtain the CDS sequence of the target antigen Ag as the target gene, and insert the target gene with restriction sites into the pET28a plasmid vector to obtain the recombinant plasmid vector pET28a-Ag; Ag represents myositis and myasthenia gravis autoantibody corresponding One of the target antigens;
[0094] Step 1.1, obtaining the CDS sequence of the autoantibody target antigen Ag by artificial synthesis or PCR method as the target gene, and adding NheI / NotI restriction sites at both ends of the target gene;
[0095] Step...
Embodiment 1
[0128]This example discloses a method for preparing myositis and myasthenia gravis autoantibody detection materials in human body fluid. The target antigen in this example is the target antigen MDA5 of myositis disease, and the negative control antigen is GAPDH. The preparation method is specific include:
[0129] 1. Plasmid construction:
[0130] Obtain the CDS sequences of autoantibody target antigens MDA5 and GAPDH by artificial synthesis or PCR method as the target gene, and add NheI / NotI restriction sites at both ends of the target gene;
[0131] Insert the target gene with a restriction site into the pET28a vector, the insertion site is NheI / NotI, to obtain a recombinant vector, which is named pET28a-MDA5 and pET28a-GAPDH, specifically:
[0132] Take the glycerol strain of the pET28a plasmid and inoculate it into 3 mL LB liquid medium resistant to kanamycin, place it in a constant temperature incubator at 37°C, and shake the bacteria overnight at 220r / min. On the secon...
Embodiment 2
[0151] This embodiment discloses a detection kit, which includes a test strip, a working solution and a blocking protein solution;
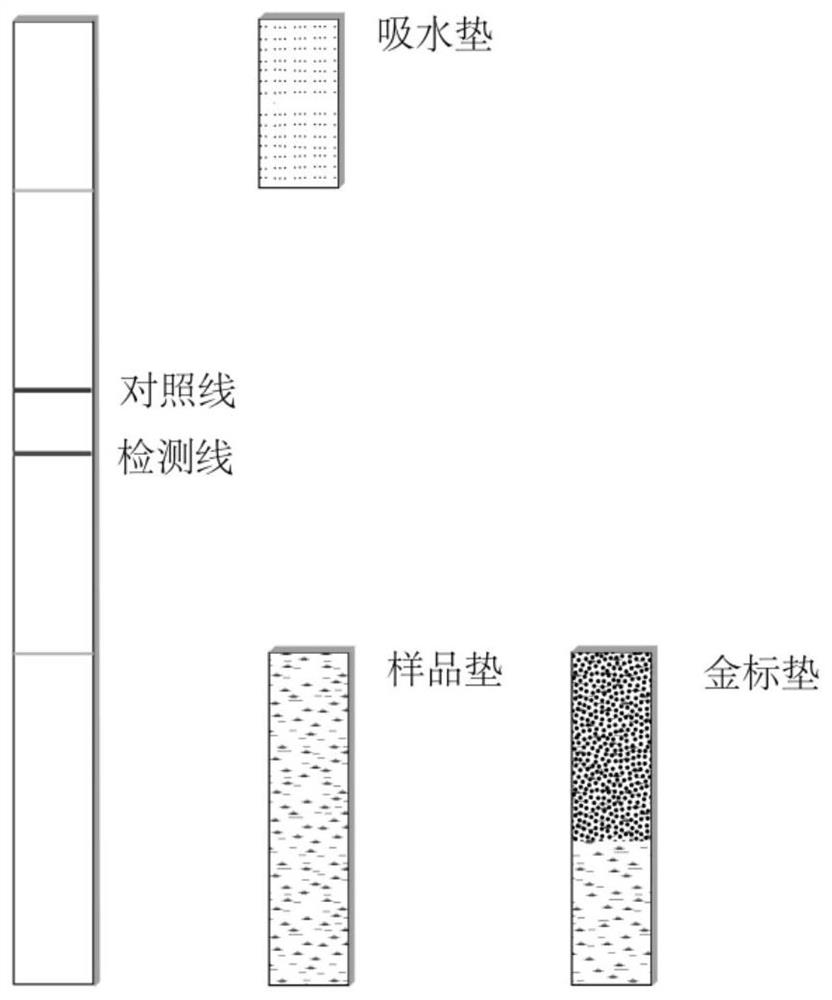
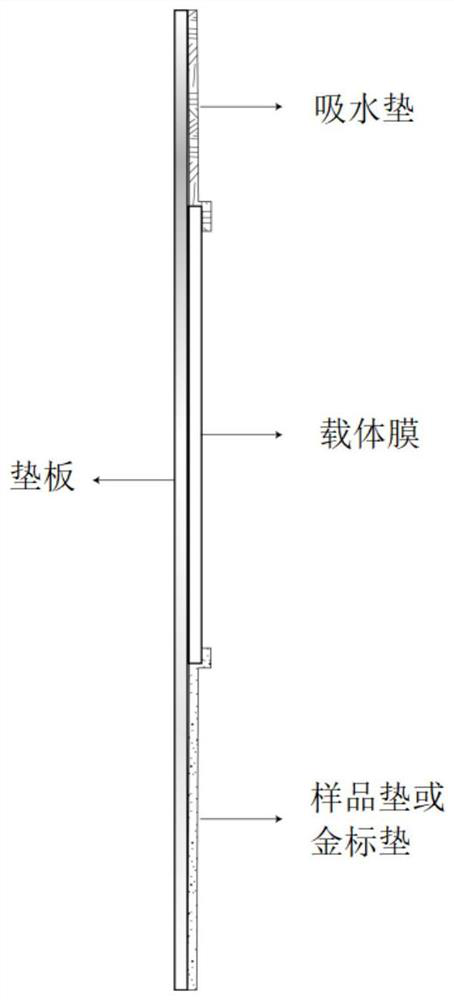
[0152] The test strip is the test strip corresponding to the target antigen in Example 1. Specifically, the test strip includes a backing plate, a carrier film with a control line and a detection line, an absorbent pad, a sample pad and a gold standard pad; the carrier film detects The target antigen Ag is immobilized on the point, and Ag represents a certain antigen in the target antigen corresponding to the autoantibody of myositis and myasthenia gravis disease; the negative control antigen is immobilized on the control line. Water-absorbing pads, carrier film, sample pads, and gold standard pads are all laid on the backing plate. figure 1 , figure 2 shown.
[0153] Wherein, the working solution is a mixed solution of 1×PBS, 0.5% Triton X-100, and 0.04% EDTA, and the pH of the working solution is 6.8.
[0154] The blocking protein solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com