Combined reactor for preparing olefin by alkane dehydrogenation and hydrocarbon catalytic cracking
A cracking reaction and reactor technology, applied in the direction of hydrocarbons, hydrocarbons, hydrocarbon cracking and hydrocarbon production, etc., can solve the problems of poor selectivity and low conversion rate of ethylene and propylene, and achieve the effect of catalytic cracking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
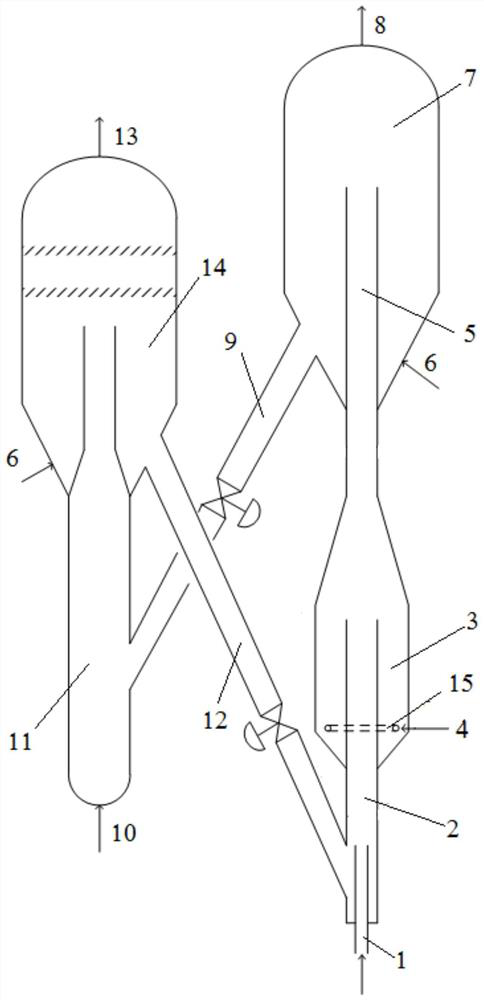
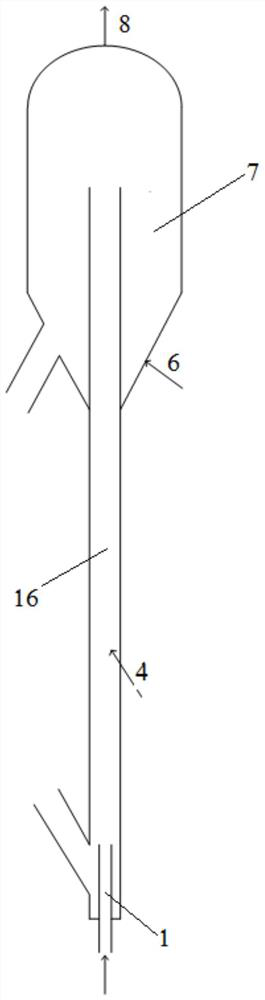
[0070] as attached figure 1 , figure 2 As shown, the reaction unit for the alkane catalytic dehydrogenation-cracking olefins provided by the application is used in conjunction with the catalyst regeneration unit. These two sets of devices can be used separately and used in conjunction with other reaction devices or catalyst regeneration devices of the prior art respectively.
[0071] like figure 1 , 2 The reaction device for catalytic dehydrogenation-cracking of alkane to produce olefins includes a reactor for catalytic dehydrogenation-cracking and a settling section 7 of the reactor. The settling section of the reactor is located at the upper part of the reactor, wherein the reactor includes Hydrogen reaction section 2 and cracking reaction section, cracking reaction section includes dense phase section 3 and dilute phase section 5, dehydrogenation reaction section 2 is positioned at the bottom of cracking reaction section, dehydrogenation reaction section 2 part stretche...
Embodiment 2
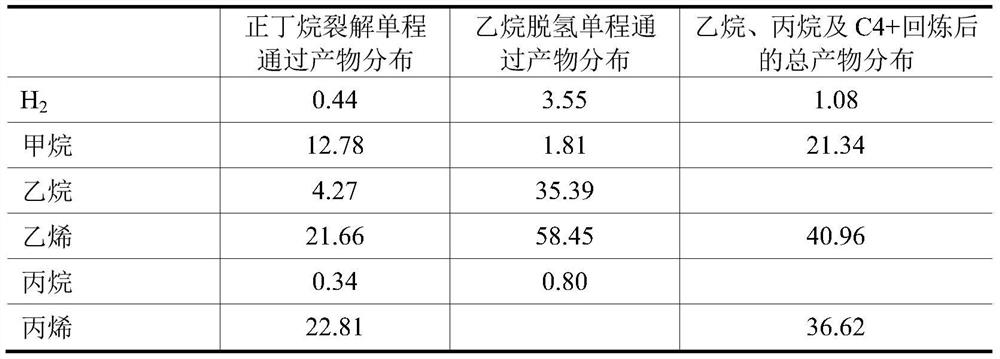
[0088] Others are the same as in Example 1, only the catalytic cracking reactor is changed into a straight tube reactor. The average superficial gas velocity and average residence time in the pyrolysis reaction zone are 10m / s and 5.5s, respectively. From the results listed in Table 2, it can be seen that the single-pass conversion rate of n-butane cracking is slightly reduced by using the straight pipe cracking reaction zone, and the selectivity of ethylene+propylene is slightly reduced, but after including ethane, propane and C4+ circulation From the point of view of the total product distribution, the yield of ethylene+propylene to fresh n-butane is still above 76%.
[0089] Table 2. The product distribution of n-butane and ethane in one pass and the product distribution of ethane, propane and C4+ after full recovery, wt%
[0090]
Embodiment 3
[0092] Others are the same as in Example 1, except that the n-butane raw material is changed into naphtha whose composition is shown in 3. The yield of naphtha catalytic cracking ethylene+propylene alone is 52.2wt%. Catalytic cracking of naphtha produces less ethane and propane, less than 3 percent. After recycling ethane and propane, the total ethylene + propylene increased by more than 2 percent.
[0093] Table 3. Naphtha composition, wt%
[0094]
[0095] Table 4. Naphtha catalytic cracking product distribution, wt%
[0096]
[0097]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com