Acne-removing composition and application thereof
A composition and anti-acne technology, which is applied in the field of daily chemicals, can solve the problems of bacterial resistance, allergic reactions of allergic skin patients, and large skin irritation, so as to reduce pigmentation and scar formation, and inhibit acne Propionibacterium acnes proliferates and the skin becomes soft and smooth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
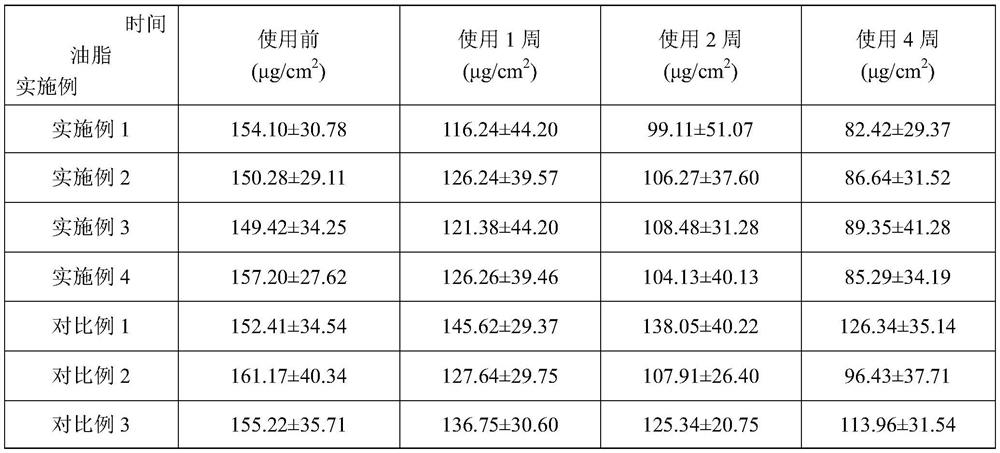
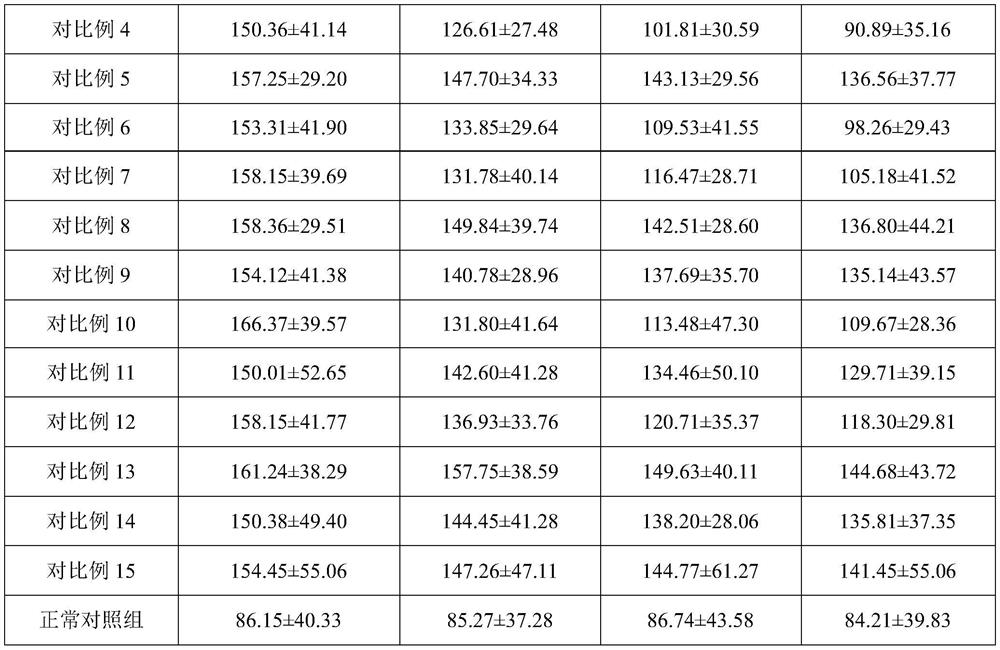
Embodiment 1
[0064] A high-efficiency, safe and mild acne-removing composition, including acne-removing active ingredients and acne-removing matrix;
[0065] The acne-removing active ingredients include: by weight, 0.5 part of linoleic acid, 0.5 part of allantoin, V A Palmitate 0.8 parts, V B6 2 parts, niacinamide 2 parts, white willow bark extract 3 parts, rhubarb extract 7 parts, scutellaria baicalensis extract 0.2 parts, coptis extract 2 parts, o-cymene-5-ol 0.05 parts, tea tree essential oil 0.02 parts , 1 part of asiaticoside, 0.3 part of bisabolol.
[0066] The acne-removing matrix includes: by weight, 0.4 parts of xanthan gum, 0.6 parts of acrylic acid (ester) / C10-30 alkanol acrylate crosslinked polymer, 0.1 part of EDTA-2Na, 5 parts of glycerin, PPG -13-decyltetradeceth-24 0.2 parts, PEG-60-hydrogenated castor oil ester 0.4 parts, triethanolamine 0.60 parts, hydroxymethyl ester 0.1 parts, essence 0.05 parts.
[0067] The preparation method of this anti-acne composition comprise...
Embodiment 2
[0075] A high-efficiency, safe and mild acne-removing composition, including acne-removing active ingredients and acne-removing matrix;
[0076] The acne-removing active ingredients include: by weight, 1.5 parts of linoleic acid, V B6 3 parts, Niacinamide 1 part, White willow bark extract 3 parts, Rhubarb extract 7 parts, Tea tree essential oil 0.02 parts, Scutellaria baicalensis extract 8 parts, Coptis chinensis extract 8 parts, V A 0.8 part of palmitate, 0.05 part of o-cymene-5-ol, 0.3 part of allantoin, 1 part of asiaticoside, 0.3 part of bisabolol.
[0077] The acne-removing matrix includes: by weight, 0.4 parts of xanthan gum, 0.6 parts of acrylic acid (ester) / C10-30 alkanol acrylate crosslinked polymer, 0.1 part of EDTA-2Na, 5 parts of glycerin, butyl 1 part of diol, 5 parts of 1.3-propanediol, 82 parts of polyethylene glycol, 0.1 part of hydroxymethyl ester, 0.2 part of PPG-13-decyltetraeth-24, 0.4 part of PEG-60-hydrogenated castor oil ester parts, essence 0.1 part....
Embodiment 3
[0086] A high-efficiency, safe and mild acne-removing composition, including acne-removing active ingredients and acne-removing matrix;
[0087] The acne-removing active ingredients include: by weight ratio, 0.05 part of linoleic acid, 0.05 part of allantoin, V A Palmitate 0.1 part, V B6 0.05 part, Niacinamide 0.1 part, White willow bark extract 1 part, Rhubarb extract 1 part, Scutellaria baicalensis extract 2 parts, Coptis chinensis extract 2 parts, O-cymene-5-ol 0.05 part, Tea tree essential oil 0.01 part , 0.05 part of bisabolol.
[0088] The acne-removing matrix includes: by weight, 0.2 parts of xanthan gum, 0.6 parts of acrylic acid (ester) / C10-30 alkanol acrylate crosslinked polymer, 0.1 part of EDTA-2Na, 3 parts of glycerin, butyl 1 part of glycol, 5 parts of 1.3-propanediol, 82 parts of polyethylene glycol, 0.1 part of hydroxymethyl ester, 0.2 part of PPG-13-decyltetraeth-24, 0.4 part of PEG-60-hydrogenated castor oil ester parts, essence 0.1 part.
[0089] The pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com