Dihydrooat alkaloid D glucoside or salt compound thereof and application of dihydrooat alkaloid D glucoside or salt compound thereof in cosmetics
A compound and cosmetic technology, applied to dihydroavenanthramide D glucoside or its salt compound and its application field in cosmetics, can solve the problems of many by-products, no occurrence, complex synthesis process, etc. Sexual dermatitis, broad application prospects, the effect of inhibiting TRPV1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
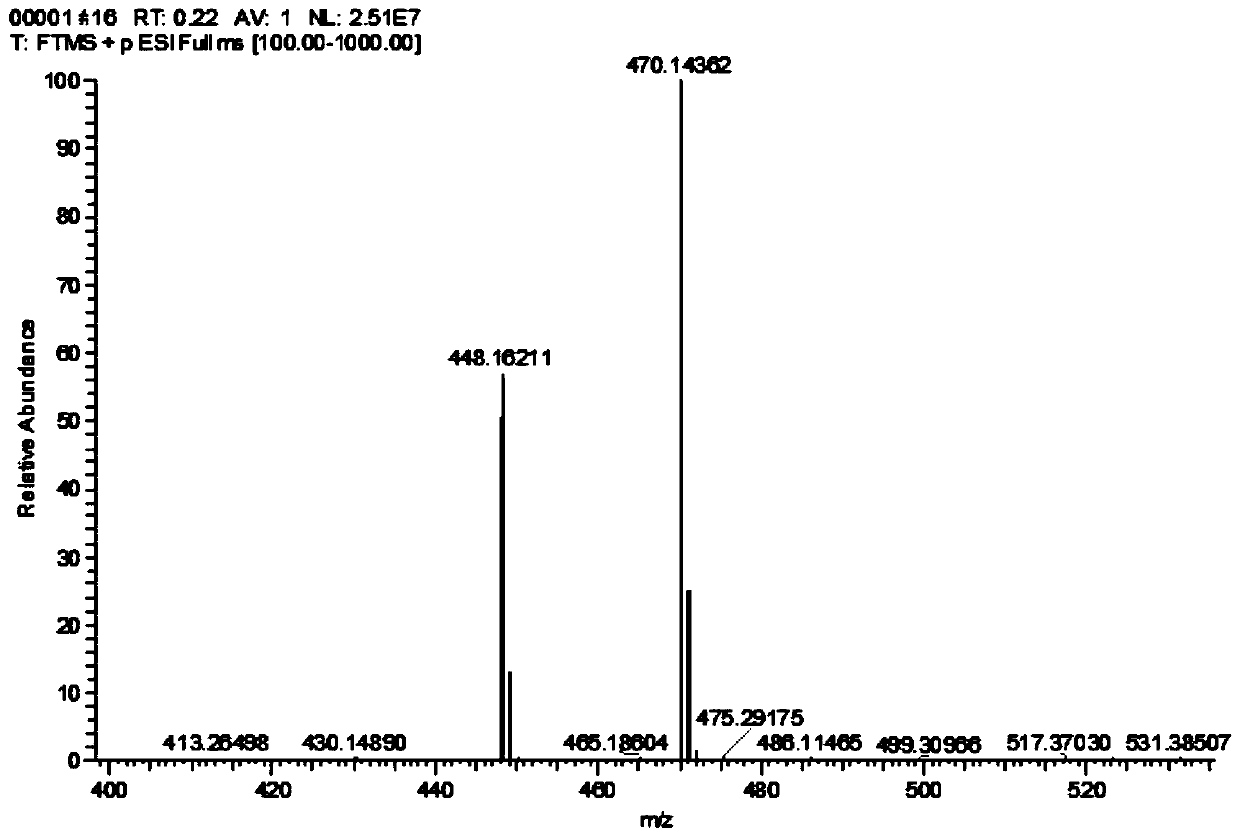
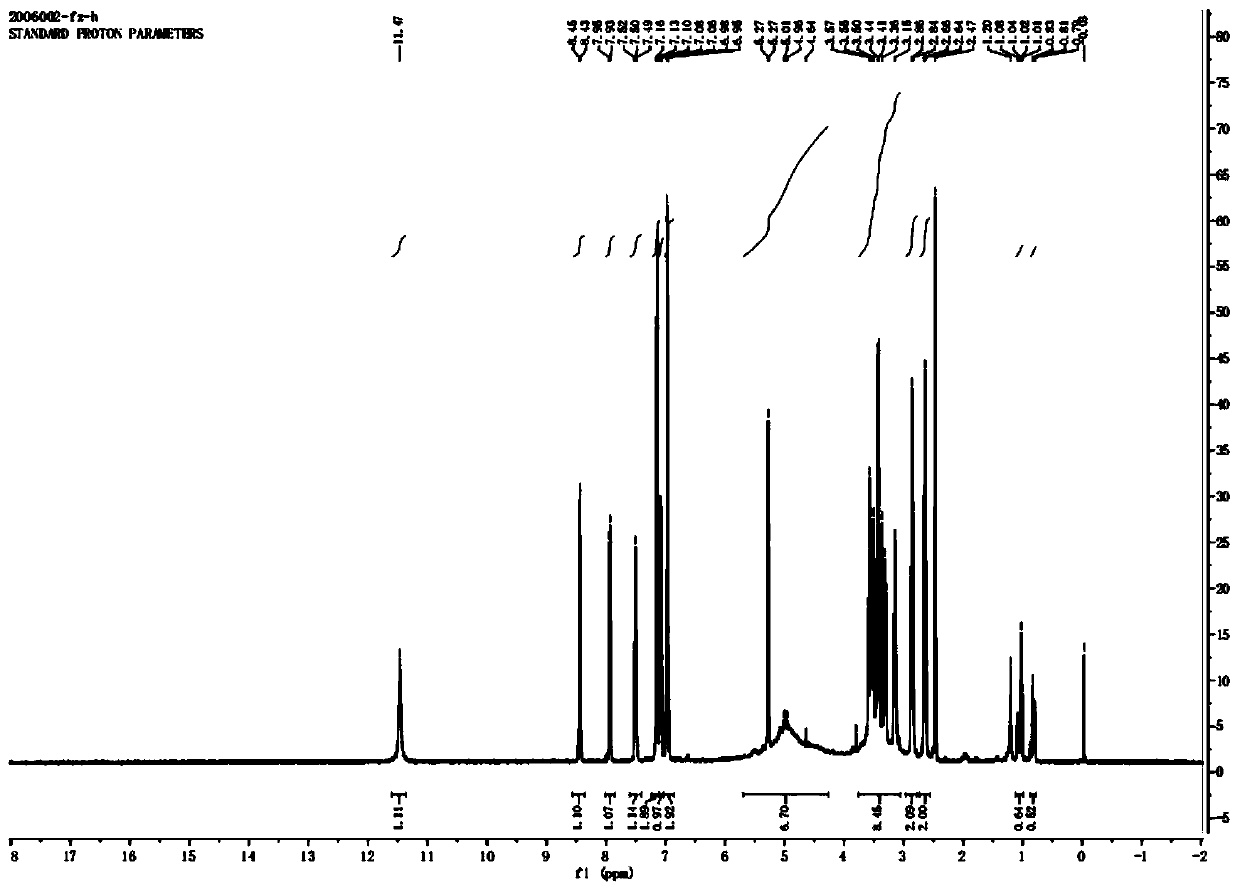
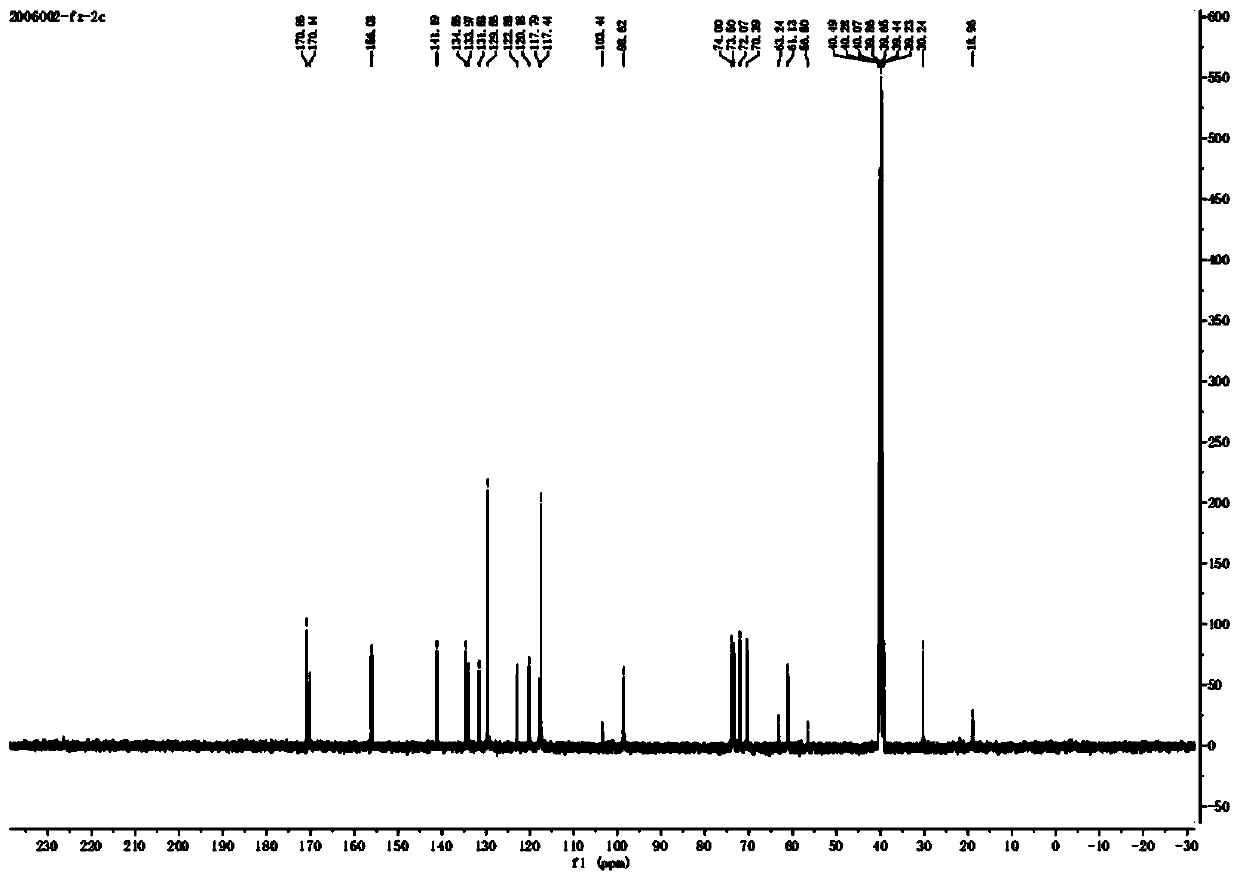
Image
Examples
preparation example Construction
[0041] The present invention provides a kind of preparation method of oat alkaloid D glucoside, comprises the following steps:
[0042] Step 1, select Leuconostoc enterocolitica NRRL-1299 (ATCC), carry out seed fermentation and shake flask culture to the strain, then centrifuge the culture solution of Leuconostoc enterococcus, completely separate the microbial cells from the enzyme liquid, and obtain the above The serum is the crude enzyme solution of dextran sucrase.
[0043] Step 2. Concentrate the dextran sucrase supernatant obtained in step 1, then dilute the residue with 20mM sodium acetate buffer solution and then concentrate to effectively remove the remaining low molecular weight components in the enzyme-containing cell culture medium to obtain purified Dextran sucrase, the tested enzyme activity is 22~30U / ml.
[0044] Step 3, configure 400ml of 500mM sodium acetate buffer solution, add 500-700g of dihydrooat alkaloid or dihydrooat alkaloid salt, 800-1200g of sucrose,...
Embodiment 1
[0054] The preparation method of dihydrooat alkaloid D glucoside is as follows:
[0055] Step 1, Leuconostoc enterococcus NRRL-1299 (ATCC), carry out seed culture to bacterial strain, culture medium formula: sucrose 10g, peptone 0.17g, Na 2 HPO 4 0.17g, 2g of agar, and sterile water to a volume of 100ml, the culture temperature was 22°C, and the culture time was 36h, and then transferred to a shake flask for culture. The shake flask culture medium formula: sucrose 5g, peptone 0.2g, Na 2 HPO 4 0.17g, dilute to 100ml with sterile water, culture at 22°C, and culture for 36 hours. Then the culture solution of Leuconostoc enterococcus is centrifuged to completely separate the microbial cells from the enzyme liquid, and the supernatant obtained is the crude enzyme liquid of dextran sucrase.
[0056] Step 2, dextran sucrase supernatant concentrates 4 times (molecular weight cut-off value is 100kDa), then the residue is diluted 4 times with the sodium acetate buffer solution of 20m...
Embodiment 2
[0063] The preparation method of dihydrooat alkaloid D potassium salt glucoside is as follows:
[0064] Step 1, Leuconostoc enterococcus NRRL-1299 (ATCC), carry out seed culture to bacterial strain, culture medium formula: sucrose 10g, peptone 0.17g, Na 2 HPO 4 0.17g, 2g of agar, and sterile water to a volume of 100ml, the culture temperature was 24°C, and the culture time was 42h, and then transferred to a shake flask for culture. The shake flask medium formula: sucrose 5g, peptone 0.2g, Na 2 HPO 4 0.17g, dilute to 100ml with sterile water, culture at 24°C, and culture for 42 hours. Then the culture solution of Leuconostoc enterococcus is centrifuged to completely separate the microbial cells from the enzyme liquid, and the supernatant obtained is the crude enzyme liquid of dextran sucrase.
[0065] Step 2, the dextran sucrase supernatant concentrates 6 times (molecular weight cut-off value is 100kDa), then the residue is diluted 4 times with the sodium acetate buffer solu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com