Method for efficiently expressing lysostaphin
A technology encoded by lysostaphin and staphylococcus enzyme, which is applied in the field of genetic engineering, can solve the problems such as the difficulty of predicting the processing and folding process of foreign proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
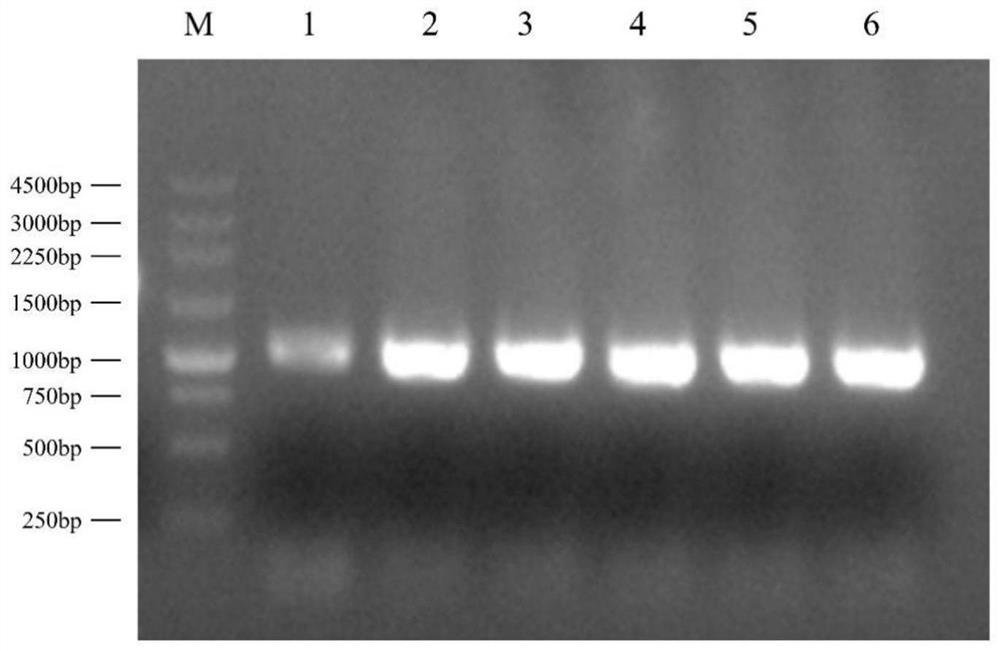
[0018] The construction of embodiment 1 lysostaphin expression bacterial strain
[0019] The lysostaphase amino acid sequence of Staphylococcus simulans (simulating Staphylococcus) was obtained from NCBI database, as shown in SEQ ID NO.1. Mature region of lysostaphin (amino acid sequence As shown in SEQ ID NO.2, the gene sequence is codon-optimized (as shown in SEQ ID NO.11), and a stop codon TAA is added at the end of the gene sequence to obtain a mature lysostaphin gene sequence, codon-optimized The gene sequence is shown in SEQ ID NO.3, SEQ ID NO.12, and SEQ ID NO.13.
[0020] Lysostaphin amino acid, SEQ ID NO.1
[0021]
[0022] Mature region of lysostaphin, SEQ ID NO.2
[0023]
[0024] Codon-optimized gene sequence: SEQ ID NO.3
[0025]
[0026]
[0027] SEQ ID NO.12
[0028]
[0029] SEQ ID NO.13
[0030]
[0031]
[0032] Taking the optimized gene sequence SEQ ID NO.3 as an example to introduce the vector construction, other gene sequences ca...
Embodiment 2
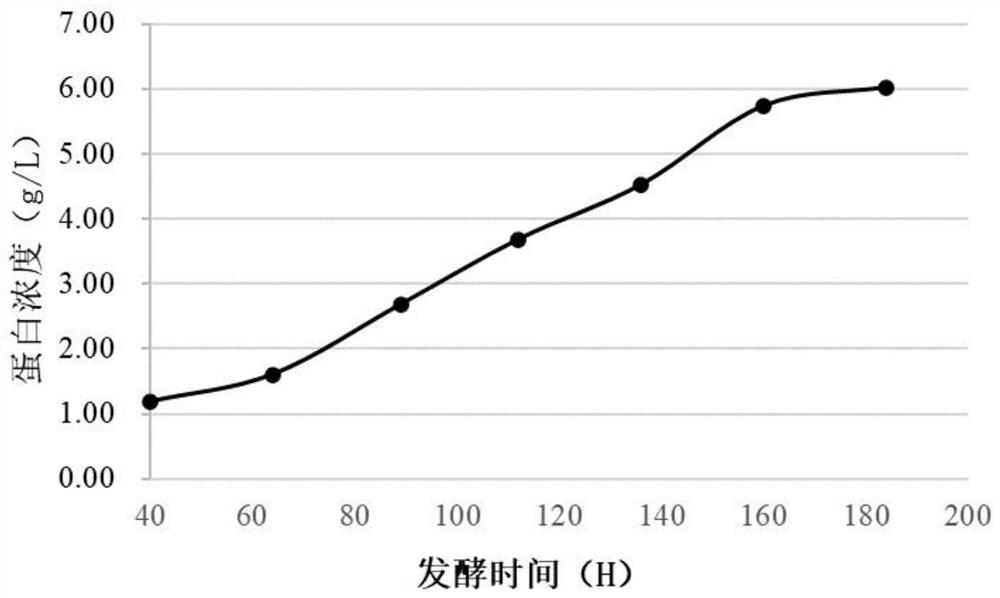
[0049] Expression and detection of embodiment 2 recombinant lysostaphin
[0050] Shake flask culture: Pick the Pichia lysostaphin expression strain constructed in Example 1, inoculate it in 5mL of LYPD medium, and culture it at 30°C and 220rpm for 24 hours to prepare a seed solution. Inoculate 50mL BMGY culture medium (250mL Erlenmeyer flask) according to 1% inoculum amount, cultivate at 30°C, 220rpm for 24 hours, centrifuge at 4000Xg for 5min, collect the bacteria, resuspend the bacteria in 50mL BMMY medium, cultivate at 30°C, 220rpm, every Add 1% methanol (final concentration) for 24 hours to induce, induce for 3 days, centrifuge at 4000Xg for 5min, and take the supernatant for detection.
[0051] Fermentation in a fermenter: Pick the Pichia lysostaphin expressing strain constructed in Example 1, inoculate it in 25 mL of YPD medium, and culture it at 30° C. at 220 rpm for 24 hours to prepare a primary seed solution. Inoculate 20 mL of primary seed liquid into 200 mL of BMGY...
Embodiment 3
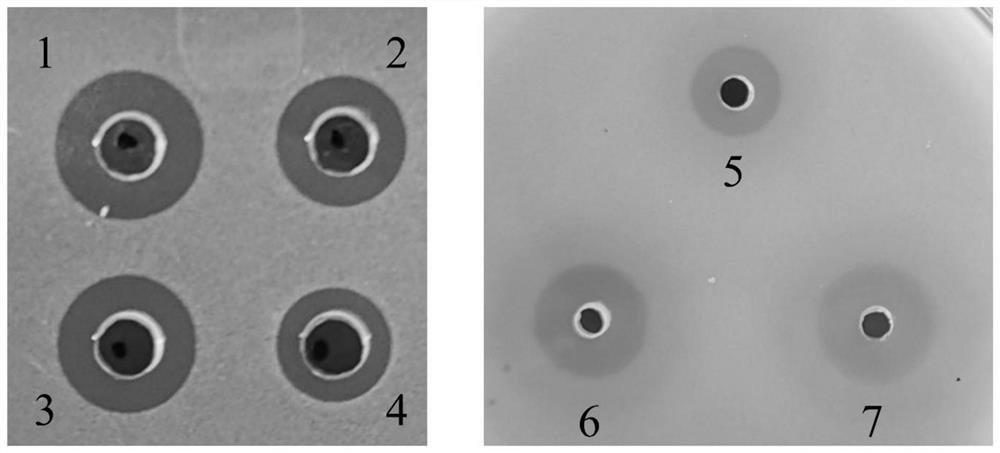
[0056] Embodiment 3 stability evaluation
[0057] Strain stability was evaluated by serial subculture. The Pichia cell colony expressing lysostaphin constructed in Example 1 was picked and inoculated in 3 mL of YPD medium, and cultured at 30° C. and 220 rpm for 24 hours to prepare a seed solution. Take 0.5mL seed solution and inoculate in 25mL BMGY medium, and culture at 30°C and 220rpm for 24 hours. Transfer the bacterial solution to a 50mL centrifuge tube, centrifuge (4000×g, room temperature) to discard the supernatant, resuspend the bacterial cells with 25mL BMMY medium with a methanol concentration of 1%, transfer the bacterial solution to a 250mL Erlenmeyer flask, 30°C, 220rpm Incubate for 72 hours, adding methanol to a concentration of 1% every 24 hours. Centrifuge (4000×g, room temperature) to take the fermentation supernatant for detection of inhibition zone and protein concentration. The selected strains were selected and subcultured for 10 generations, and the in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com