Environment-friendly preparation method of substituted oxazole compound
A compound, the technology of oxazole, which is applied in the field of environmentally friendly preparation of oxazole compounds, can solve the problems of large amount of waste water, high product cost, unfavorable environmental protection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
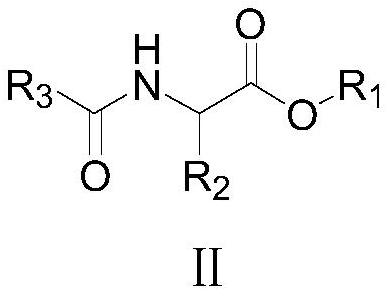
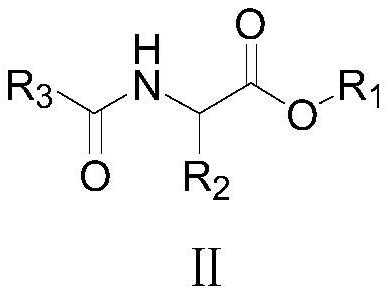
[0054] Example 1: 4-methyl-5-ethoxy-2-ethoxycarbonyl oxazole (Ⅰ 1 ) preparation
[0055] To a 250 ml flask was added 100 g of chloroform, 33.3 g (0.1 mol) of triphenylphosphine dichloride and 21.7 g (0.1 mol) of N-ethoxyoxalyl-α-alanine ethyl ester, 20-25 At ℃, add 20.2 grams (0.2 moles) of triethylamine dropwise within 2 hours, then react at 35-40℃ for 1 hour, check that the reaction of raw materials is complete, add 30 grams of water, separate layers, and extract the obtained water layer twice with chloroform (Using 30 grams in total), the organic phases are combined, and the organic phase atmospheric distillation reclaims chloroform, and then underpressure distillation obtains 18.8 grams of 4-methyl-5-ethoxyl-2-ethoxycarbonyloxazole, yield 94.4 %, GC purity 99.9%; the main component of the residue after vacuum distillation is triphenylphosphine oxide, which can be used repeatedly as a dehydrating agent.
[0056] The NMR data of the resulting product are as follows:
[00...
Embodiment 2
[0059] Example 2: 4-methyl-5-ethoxy-2-ethoxycarbonyl oxazole (Ⅰ 1 ) preparation
[0060] In 500 milliliters of four-necked flasks, add 100 grams of toluene, 3.4 grams (0.01 moles) triphenylphosphine dichloride, 21.7 grams (0.1 moles) N-ethoxy oxalyl-α-alanine ethyl ester, 20.8 grams ( 0.206 moles) of triethylamine, dropwise add a solution of 9.9 grams (0.1 moles) of phosgene and 50 grams of toluene between 25-30°C, dropwise addition is completed in 2 hours, then react at 65-70°C for 1 hour, and detect that the reaction of raw materials is complete. Add 30 grams of water, separate layers, and the gained water layer is extracted twice with toluene (using 30 grams in total), the organic phases are combined, and the organic phase is distilled under normal pressure to reclaim toluene, and then distilled under reduced pressure to obtain 4-methyl-5-ethoxy - 18.9 g of 2-ethoxycarbonyloxazole, yield 94.9%, GC purity 99.9%; the main component of the residue after vacuum distillation is...
Embodiment 3
[0061] Example 3: 4-methyl-5-ethoxy-2-ethoxycarbonyl oxazole (Ⅰ 1 ) preparation
[0062] Add 100 g of toluene, 27.8 g (0.1 mole) of triphenylphosphine oxide, 21.7 g (0.1 mole) of N-ethoxyoxalyl-α-alanine ethyl ester into a 500 ml four-neck flask, at 20-25°C , add dropwise a solution of 100 grams of toluene and 9.9 grams (0.033 moles) of triphosgene to the above-mentioned 500 milliliter four-necked flask within 2 hours, and dropwise add 24.3 grams (0.24 moles) of triethylamine at the same time. 1 hour, detection raw material reaction is complete, adds 30 grams of water, layering, gained water layer is extracted twice with toluene (uses 30 grams altogether), merges organic phase, organic phase normal pressure distillation reclaims toluene, then underpressure distillation obtains 4- Methyl-5-ethoxy-2-ethoxycarbonyloxazole 19.1 g, yield 96%, GC purity 99.9%; the main component of the residue after vacuum distillation is triphenylphosphine oxide, which can be used repeatedly as a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com