A kind of breast milk source Pediococcus pentosacea and its application
A technology for Pediococcus pentosaceus and breast milk, which is applied to Pediococcus pentosaceus from breast milk and its application field, can solve the problems of poor bacteriostatic effect and narrow bacteriostatic spectrum, and achieves good gastrointestinal tolerance and strong bacteriostatic activity. , the effect of improving immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Isolation and identification of Pediococcus pentosaceus HM75-1 derived from human milk
[0033] 1.1 Separation and purification
[0034] The breast milk samples (breast milk of Uyghur women in Kashgar, Xinjiang) were diluted with sterile water, and the dilution gradient was 10 -3 、10 -4 、10 -5 ; MRS medium (containing cysteine 0.5g / L) and Lactobacillus selective medium (LB medium) are sterilized and poured onto the plate, and after coagulation, 100 μL of breast milk samples of different dilution gradients are sucked and spread on the plate, Number and record, and invert for 24-48 hours in an anaerobic box at 37°C.
[0035] Select an appropriate gradient plate for each sample to pick 15-20 single colonies with different color, size and shape to observe the cell morphology, streak the colonies with rod-shaped or short rod-shaped cells for anaerobic culture, and repeatedly streak to The color, size and shape of the colonies in the same plate are consistent....
Embodiment 2
[0059] Example 2 Bacteriostatic properties of human milk source Pediococcus pentosaceus HM75-1
[0060] 2.1 Determination of antibacterial activity of strain HM75-1
[0061] Activation of strains: Take Pediococcus pentosaceus HM75-1 from breast milk that has been preserved in advance and ablate it, insert 1% into the sterilized MRS culture medium in an aseptic operating table, and incubate anaerobically at 37°C for 32 hours. Make the bacterial liquid in the logarithmic growth phase; take the food-borne pathogens preserved in advance (enteropathogenic Escherichia coli (CICC 10411), Salmonella enterica serotype Typhimurium (CICC 10420), Enterotoxigenic Escherichia coli (CICC 10421), Enterohaemorrhagic Escherichia coli (CICC 21530), Listeria monocytogenes (CGMCC 1.9136), Salmonella enterica subsp. enterica (CGMCC 1.10754) , ablated, put 1% into sterilized LB culture medium in a sterile operating table, and incubated at 37°C for 24 hours. Make the bacterial liquid in the logarit...
Embodiment 3
[0071] Example 3 Optimization of culture conditions for human milk-derived Pediococcus pentosaceus HM75-1
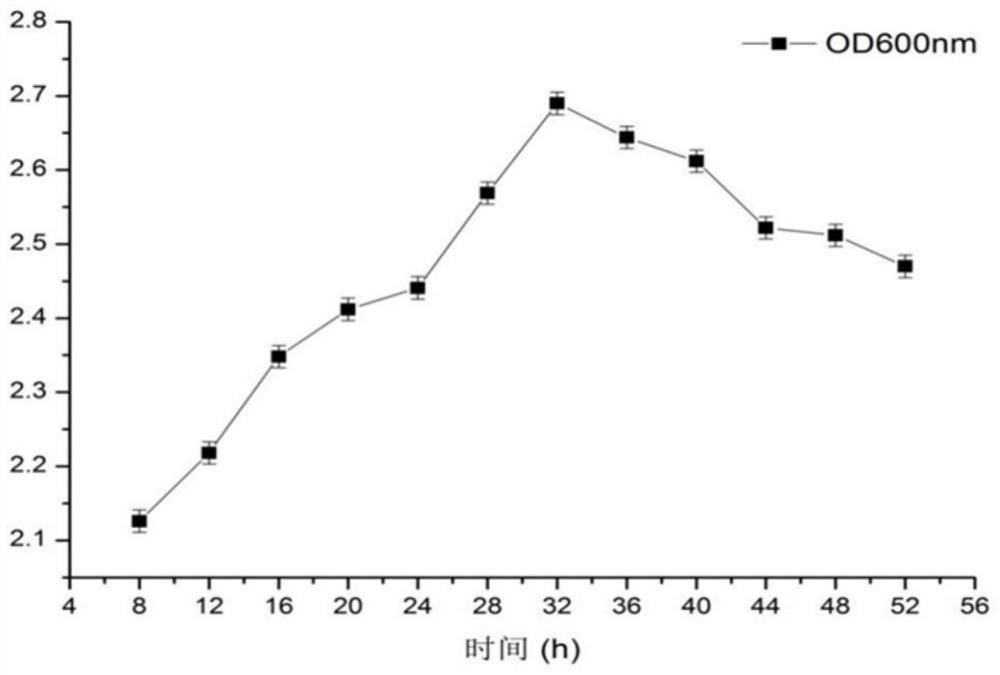
[0072] 3.1 Effect of culture time on the antibacterial activity of strain HM75-1
[0073] Put the activated human milk-derived Pediococcus pentosaceus HM75-1 into the prepared MRS liquid medium according to the 10% inoculation amount, place it in an anaerobic incubator at 37°C for 52 hours, and take the bacterial solution every 4 hours. The growth curve is drawn by measuring the OD600nm value and pH value, the fermentation broth at each time point is centrifuged at 8000r / min for 15min, and the supernatant is collected for subsequent use; draw 150 μL of activated enterotoxigenic Escherichia coli (CICC 10421) bacteria suspension evenly spread on LB medium, let it stand for 30 minutes, and then put 4 sterilized Oxford cups (Oxford cup inner diameter is 8mm), and inject 200 μL of breast milk-derived pentose tablets at different time points into 3 of them One of the fermenta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com