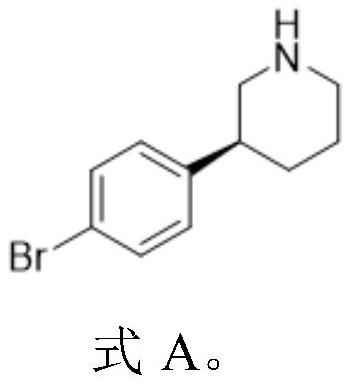
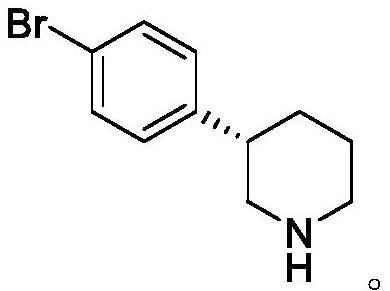
Preparation method of niraparib intermediate (S)-3-(4-bromophenyl) piperidine
A technology of bromophenyl and intermediate, which is applied in the field of preparation of niraparib intermediate-3-piperidine, can solve the problems of expensive preparation cost of raw materials and reagents, poor operation safety and high risk factor, and achieve industrialized amplification The effect of production, overall cost reduction, and safety hazards reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
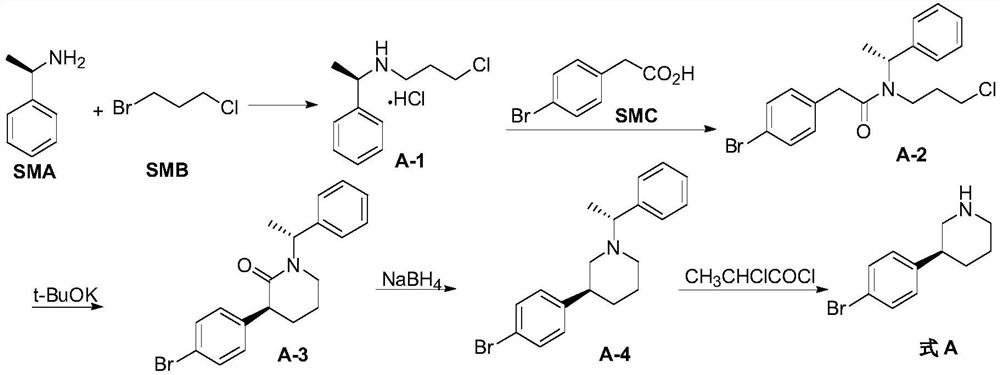
[0090] Embodiment 1, the preparation of compound A-1
[0091] Add 156g of the raw material compound SMB and 780ml of acetonitrile into the reaction vessel, cool down to 0-10°C and add 264g of the raw material compound SMA dropwise, after the addition is completed, heat up to 20-30°C, keep stirring at this temperature for 14-16h; add ethyl acetate to the reaction solution Extract the ester, combine the organic layers, add 1M hydrochloric acid to wash, then add concentrated hydrochloric acid to precipitate a solid, filter with suction, wash the filter cake with ethyl acetate, and dry it with air at 50-60°C for 10-12h to obtain compound A-1 (163g, harvested rate 70%);
[0092] Embodiment 1 On the basis of the above-mentioned experiments, the optimization condition experiment also includes:
[0093] The solvent is the optimized parallel experiment of DMF and THF;
[0094] Optimized parallel experiments with reaction temperatures of 40-50°C and 0-10°C;
[0095] The optimized par...
Embodiment 2
[0096] Embodiment 2, the preparation of compound A-2
[0097] Add 160g of compound A-1, 1.6L of dichloromethane and 147g of the raw material compound SMC into the reaction vessel, cool the reaction solution to 0-10°C, add 69g of triethylamine, then add 144g of condensing agent CDI in batches, and raise the temperature of the reaction solution Stir the reaction at 20-30°C for 10-12h; add water to the reaction liquid to quench, add dilute hydrochloric acid to adjust pH=1-2, remove the water phase, and concentrate the organic phase under reduced pressure to remove the dichloromethane solvent to obtain compound A-2 ( 263g, yield 98%);
[0098] Embodiment 2 On the basis of the above-mentioned experiments, the optimization condition experiment also includes:
[0099]The base is DIPEA, NaHCO 3 Optimized parallel experiments;
[0100] Parallel experiments were optimized for THF as the organic solvent.
Embodiment 3
[0101] Embodiment 3, the preparation of compound A-3
[0102] Add 125g potassium tert-butoxide and 450ml solvent DMF to the reaction vessel, add 220g compound A-2 (solution dissolved in 450ml DMF) after the reaction solution is cooled to 0-10°C, and the reaction solution is heated to 20-30°C and stirred for 10 -12h; After treatment, add water and ethyl acetate to extract, combine the organic layers, wash with water, concentrate under reduced pressure to remove ethyl acetate, add petroleum ether to the residue, stir and filter with suction, and dry the solid at 40-50°C with air blast to obtain compound A-3 (76g , yield 38%) (equivalent to 76% of theoretical yield);
[0103] Embodiment 3 On the basis of the above-mentioned experiments, the optimization condition experiment also includes:
[0104] Solvent is the optimized parallel experiment of THF;
[0105] Optimized parallel experiments with reaction temperatures of 30-40°C and 0-10°C;
[0106] The bases are optimized parall...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com